Silver Jubilee of Stroke Thrombolysis With Alteplase: Evolution of the Therapeutic Window
- PMID: 33732203
- PMCID: PMC7956989
- DOI: 10.3389/fneur.2021.593887
Silver Jubilee of Stroke Thrombolysis With Alteplase: Evolution of the Therapeutic Window
Abstract
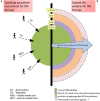
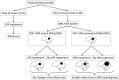
In 1995, the results of a landmark clinical trial by National Institute of Neurological Disorders and Stroke (NINDS) made a paradigm shift in managing acute cerebral ischemic stroke (AIS) patients at critical care centers. The study demonstrated the efficacy of tissue-type plasminogen activator (tPA), alteplase in improving neurological and functional outcome in AIS patients when administered within 3 h of stroke onset. After about 12 years of efforts and the results of the ECASS-III trial, it was possible to expand the therapeutic window to 4.5 h, which still represents a major logistic issue, depriving many AIS patients from the benefits of tPA therapy. Constant efforts in this regards are directed toward either speeding up the patient recruitment for tPA therapy or expanding the current tPA window. Efficient protocols to reduce the door-to-needle time and advanced technologies like telestroke services and mobile stroke units are being deployed for early management of AIS patients. Studies have demonstrated benefit of thrombolysis guided by perfusion imaging in AIS patients at up to 9 h of stroke onset, signifying "tissue window." Several promising pharmacological and non-pharmacological approaches are being explored to mitigate the adverse effects of delayed tPA therapy, thus hoping to further expand the current tPA therapeutic window without compromising safety. With accumulation of scientific data, stroke organizations across the world are amending/updating the clinical recommendations of tPA, the only US-FDA approved drug for managing AIS patients. Alteplase has been a part of our neurocritical care and we intend to celebrate its silver jubilee by dedicating this review article discussing its journey so far and possible future evolution.
Keywords: acute cerebral ischemic stroke; alteplase; neurocritical care; stroke; thrombolysis (tPA).
Copyright © 2021 Pan and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. . 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. (2018) 49:e46–e110. 10.1016/j.jvs.2018.04.007 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

