A Novel DNA Vaccine Against SARS-CoV-2 Encoding a Chimeric Protein of Its Receptor-Binding Domain (RBD) Fused to the Amino-Terminal Region of Hepatitis B Virus preS1 With a W4P Mutation
- PMID: 33732258
- PMCID: PMC7959807
- DOI: 10.3389/fimmu.2021.637654
A Novel DNA Vaccine Against SARS-CoV-2 Encoding a Chimeric Protein of Its Receptor-Binding Domain (RBD) Fused to the Amino-Terminal Region of Hepatitis B Virus preS1 With a W4P Mutation
Abstract
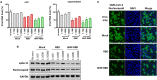
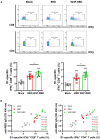
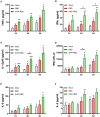
A coronavirus SARS-CoV-2, which has caused the pandemic viral pneumonia disease COVID-19, significantly threatens global public health, highlighting the need to develop effective and safe vaccines against its infection. In this study, we developed a novel DNA vaccine candidate against SARS-CoV-2 by expressing a chimeric protein of its receptor-binding domain (RBD) fused to a 33-bp sequence (11 aa) from the hepatitis B virus (HBV) preS1 region with a W4P mutation (W4P-RBD) at the N-terminal region and evaluated its immunogenicity. In vitro transfection experiments in multiple cell lines demonstrated that W4P-RBD vs. wild-type RBD protein (W-RBD) led to enhanced production of IL-6 and TNFα at the transcription and translation levels, suggesting the adjuvant potential of N-terminal HBV preS1 sequences for DNA vaccines against SARS-CoV-2. W4P-RBD also led to enhanced production of IgG and IgA, which can neutralize and block SARS-CoV-2 infection in both blood sera and bronchoalveolar lavage (BAL) fluid from the lung in vaccinated mice. Additionally, W4P-RBD led to an enhanced T-cell-mediated cellular immune response under S1 protein stimulation. In summary, W4P-RBD led to robust humoral and cell-mediated immune responses against SARS-CoV-2 in vaccinated mice, highlighting its feasibility as a novel DNA vaccine to protect against SARS-CoV-2 infection.
Keywords: COVID-19; DNA vaccine; HBV preS1; Receptor-binding domain (RBD); SARS-CoV-2; W4P-RBD.
Copyright © 2021 Jeong, Choi, Seo and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

