Cost-effectiveness analysis of different sequences of osimertinib administration for epidermal growth factor receptor-mutated non-small-cell lung cancer
- PMID: 33732316
- PMCID: PMC7903425
- DOI: 10.3892/etm.2021.9774
Cost-effectiveness analysis of different sequences of osimertinib administration for epidermal growth factor receptor-mutated non-small-cell lung cancer
Abstract
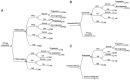
Osimertinib is a third-generation epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) that is clinically effective in patients with EGFR-mutated non-small-cell lung cancer (NSCLC). However, the use of this treatment is limited by its high cost. A cost-effectiveness analysis of different sequences of osimertinib administration in China and the United States was conducted in the present study. Markov models were established based on data from the FLAURA and AURA3 trials. First-line osimertinib was compared with both first-generation EGFR-TKIs and second-line osimertinib after the failure of first-generation EGFR-TKIs. The analysis also considered different payment modalities available in China. Additionally, one-way and probability sensitivity analyses, with a willingness-to-pay threshold (WTP) of three times the per capita gross domestic product [$27,783/quality-adjusted life year (QALY) for China and $100,000/QALY for the United States], were performed. The first-line osimertinib group displayed higher QALYs and costs than those of the first-generation EGFR-TKI group. The first generation EGFR-TKI group displayed an incremental cost-effectiveness ratio (ICER) of $212,252/QALY in China and $151,922/QALY in the United States. In addition, the ICERs were negative in the second-line osimertinib group, with higher QALYs and lower costs compared with those in the first-line osimertinib group. Furthermore, osimertinib company donation was of benefit in China, with an average cost-effectiveness of $836/QALY. The one-way sensitivity analysis highlighted the influence of utilities in different states. First-line osimertinib could be cost-effective either with higher WTP or a price reduction of 68% in China and 9% in the United States. Although first-line osimertinib therapy could have health benefits, it was not cost-effective compared with first-line first-generation EGFR-TKIs and second-line osimertinib therapy. However, paying via company donation may be a good choice in China.
Keywords: EGFR-TKI; NSCLC; QALY; cost-effectiveness; osimertinib.
Copyright: © Li et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
