Curcumin inhibits the growth of liver cancer by impairing myeloid-derived suppressor cells in murine tumor tissues
- PMID: 33732362
- PMCID: PMC7905673
- DOI: 10.3892/ol.2021.12547
Curcumin inhibits the growth of liver cancer by impairing myeloid-derived suppressor cells in murine tumor tissues
Abstract
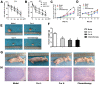
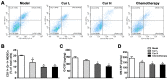
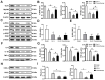
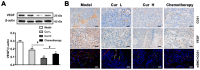
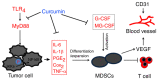
Curcumin, one of the active ingredients of Curcuma longa (Jianghuang), has been reported to exert multiple bioactivities, including pro-apoptotic and anti-inflammatory activities. In recent years, curcumin has been extensively studied, and it has been revealed that curcumin inhibits the growth of numerous types of cancer. However, to the best of our knowledge, the inhibitory effects of curcumin on the activation or expansion of myeloid-derived suppressor cells (MDSCs) in liver cancer and the underlying mechanism have not yet been determined. Therefore, the present study aimed to investigate the inhibitory effect of curcumin on MDSC activity and the associated anti-neoplastic mechanism in a HepG2 ×enograft mouse model. The effect of curcumin on the viability of Huh-7, MHCC-97H and HepG2 cells in vitro was analyzed using a Cell Counting Kit-8 assay. The effects of curcumin on tumor growth, numbers of MDSCs, expression levels of proteins involved in the toll-like receptor 4 (TLR4)/NF-κB signaling pathway, levels of related inflammatory factors and angiogenesis were determined in HepG2 ×enograft model mice, which were given different doses of curcumin via intragastrical administration. The results of the present study revealed that curcumin inhibited the viability of Huh-7, MHCC-97H and HepG2 cells and the growth of HepG2 ×enograft tumors in mice. Flow cytometric analysis indicated that curcumin reduced the number of MDSCs in mouse xenograft tumors. In addition, the results demonstrated that curcumin inhibited the TLR4/NF-κB signaling pathway and the expression of inflammatory factors, including IL-6, IL-1β, prostaglandin E2 and cyclooxygenase-2, in mouse xenograft tumors. Furthermore, curcumin suppressed the secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte-colony stimulating factor (G-CSF), which are essential factors for MDSCs modulation, in tumor tissues. Additionally, curcumin was revealed to inhibit angiogenesis, which was demonstrated by the downregulation of the expression levels of vascular endothelial growth factor, CD31 and α-smooth muscle actin in western blotting, immunohistochemistry and immunofluorescence experiments. In conclusion, the findings of the present study identified a novel mechanism via which curcumin may suppress the growth of liver cancer by reducing the numbers of MDSCs and subsequently disrupting the process of angiogenesis. These conclusions were supported by the observed inactivation of the TLR4/NF-κB signaling pathway-mediated inflammatory response and the downregulation of GM-CSF and G-CSF secretion in xenograft tissues.
Keywords: curcumin; liver cancer; myeloid-derived suppressor cells; toll-like receptor 4/NF-κB; vascular endothelial growth factor.
Copyright: © Tian et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. - DOI - PubMed
-
- De Sanctis F, Solito S, Ugel S, Molon B, Bronte V, Marigo I. MDSCs in cancer: Conceiving new prognostic and therapeutic targets. Biochim Biophys Acta. 2016;1865:35–48. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
