Regeneration of critical-sized defects, in a goat model, using a dextrin-based hydrogel associated with granular synthetic bone substitute
- PMID: 33732486
- PMCID: PMC7947577
- DOI: 10.1093/rb/rbaa036
Regeneration of critical-sized defects, in a goat model, using a dextrin-based hydrogel associated with granular synthetic bone substitute
Abstract
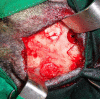
The development of injectable bone substitutes (IBS) have obtained great importance in the bone regeneration field, as a strategy to reach hardly accessible defects using minimally invasive techniques and able to fit to irregular topographies. In this scenario, the association of injectable hydrogels and bone graft granules is emerging as a well-established trend. Particularly, in situ forming hydrogels have arisen as a new IBS generation. An in situ forming and injectable dextrin-based hydrogel (HG) was developed, aiming to act as a carrier of granular bone substitutes and bioactive agents. In this work, the HG was associated to a granular bone substitute (Bonelike®) and implanted in goat critical-sized calvarial defects (14 mm) for 3, 6 and 12 weeks. The results showed that HG improved the handling properties of the Bonelike® granules and did not affect its osteoconductive features, neither impairing the bone regeneration process. Human multipotent mesenchymal stromal cells from the umbilical cord, extracellular matrix hydrolysates and the pro-angiogenic peptide LLKKK18 were also combined with the IBS. These bioactive agents did not enhance the new bone formation significantly under the conditions tested, according to micro-computed tomography and histological analysis.
Keywords: Bonelike®; bone regeneration; calvarial defect; granular ceramics; injectable hydrogel; polysaccharide.
© The Author(s) 2020. Published by Oxford University Press.
Figures




Similar articles
-
In vivo systemic toxicity assessment of an oxidized dextrin-based hydrogel and its effectiveness as a carrier and stabilizer of granular synthetic bone substitutes.J Biomed Mater Res A. 2019 Aug;107(8):1678-1689. doi: 10.1002/jbm.a.36683. Epub 2019 Apr 12. J Biomed Mater Res A. 2019. PMID: 30920095
-
The application of Bonelike® Poro as a synthetic bone substitute for the management of critical-sized bone defects - A comparative approach to the autograft technique - A preliminary study.Bone Rep. 2021 Apr 9;14:101064. doi: 10.1016/j.bonr.2021.101064. eCollection 2021 Jun. Bone Rep. 2021. PMID: 33981810 Free PMC article.
-
Inflammatory response to dextrin-based hydrogel associated with human mesenchymal stem cells, urinary bladder matrix and Bonelike® granules in rat subcutaneous implants.Biomed Mater. 2016 Oct 27;11(6):065004. doi: 10.1088/1748-6041/11/6/065004. Biomed Mater. 2016. PMID: 27786165
-
Dental pulp stem cells and Bonelike® for bone regeneration in ovine model.Regen Biomater. 2019 Feb;6(1):49-59. doi: 10.1093/rb/rby025. Epub 2018 Dec 22. Regen Biomater. 2019. PMID: 30740242 Free PMC article.
-
Synthesis and characterization of chitosan-silicate hydrogel as resorbable vehicle for bonelike-bone graft.J Nanosci Nanotechnol. 2009 Jun;9(6):3714-9. doi: 10.1166/jnn.2009.ns56. J Nanosci Nanotechnol. 2009. PMID: 19504908
Cited by
-
Recent advances in regenerative biomaterials.Regen Biomater. 2022 Dec 5;9:rbac098. doi: 10.1093/rb/rbac098. eCollection 2022. Regen Biomater. 2022. PMID: 36518879 Free PMC article. Review.
-
Randomized clinical study of injectable dextrin-based hydrogel as a carrier of a synthetic bone substitute.Clin Oral Investig. 2023 Mar;27(3):979-994. doi: 10.1007/s00784-023-04868-9. Epub 2023 Jan 28. Clin Oral Investig. 2023. PMID: 36707442 Free PMC article. Clinical Trial.
-
Effects of Scaffold Shape on Bone Regeneration: Tiny Shape Differences Affect the Entire System.ACS Nano. 2022 Aug 23;16(8):11755-11768. doi: 10.1021/acsnano.2c03776. Epub 2022 Jul 14. ACS Nano. 2022. PMID: 35833725 Free PMC article.
-
3D printing materials and 3D printed surgical devices in oral and maxillofacial surgery: design, workflow and effectiveness.Regen Biomater. 2024 Jun 27;11:rbae066. doi: 10.1093/rb/rbae066. eCollection 2024. Regen Biomater. 2024. PMID: 39169972 Free PMC article. Review.
-
Injectable and Cell-Laden Hydrogel in the Contained Bone Defect Animal Model: A Systematic Review.Tissue Eng Regen Med. 2023 Oct;20(6):829-837. doi: 10.1007/s13770-023-00569-2. Epub 2023 Aug 10. Tissue Eng Regen Med. 2023. PMID: 37563482 Free PMC article.
References
-
- Stevens MM. Biomaterials for bone tissue engineering. Mater Today 2008;11:18–25.
-
- Amini AA, Nair LS. Injectable hydrogels for bone and cartilage repair. Biomed Mater 2012;7:024105. - PubMed
-
- Bohner M. Resorbable biomaterials as bone graft substitutes. Mater Today 2010;13:24–30.
-
- Lopes M, Knowles J, Santos J et al. Direct and indirect effects of P2O5 glass reinforced-hydroxyapatite composites on the growth and function of osteoblast-like cells. Biomaterials 2000;21:1165–72. - PubMed
-
- Lopes M, Knowles J, Santos J. Structural insights of glass-reinforced hydroxyapatite composites by Rietveld refinement. Biomaterials 2000;21:1905–10. - PubMed
LinkOut - more resources
Full Text Sources

