Nickel-Catalyzed Dicarbofunctionalization of Alkenes
- PMID: 33732540
- PMCID: PMC7963398
- DOI: 10.1021/acscatal.0c02115
Nickel-Catalyzed Dicarbofunctionalization of Alkenes
Abstract
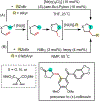
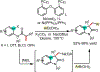
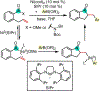
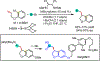
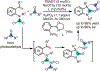
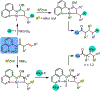
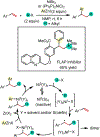
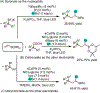
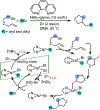
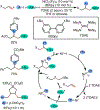
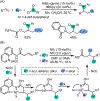
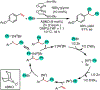
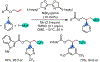
1,2-Dicarbofunctionalization of alkenes has emerged as an efficient synthetic strategy for preparing substituted molecules by coupling readily available alkenes with electrophiles and/or nucleophiles. Nickel complexes serve as effective catalysts owing to their tendency to undergo facile oxidative addition and slow β-hydride elimination, and their capability to access both two-electron and radical pathways. Two-component alkene functionalization reactions have achieved high chemo-, regio-, and stereoselectivities by tethering one of the coupling partners to the alkene substrate. Three-component reactions, however, often incorporate directing groups to control the selectivity. Only a few examples of directing-group-free difunctionalizations of unactivated alkenes have been reported. Therefore, great opportunities exist for the development of three-component difunctionalization reactions with broad substrate scopes and tunable chemo-, regio-, and stereoselectivities.
Keywords: alkenes; dicarbofunctionalization; nickel catalysis; selectivity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



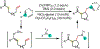


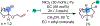


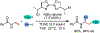
References
-
-
For selected reviews, see:
- Jensen KH; Sigman MS Mechanistic Approaches to Palladium-Catalyzed Alkene Difunctionalization Reactions. Org. Biomol. Chem 2008, 6, 4083–4088. - PMC - PubMed
- McDonald RI; Liu G; Stahl SS Palladium (II)-Catalyzed Alkene Functionalization via Nucleopalladation: Stereochemical Pathways and Enantioselective Catalytic Applications. Chem. Rev 2011, 111, 2981–3019. - PMC - PubMed
- Egami H; Sodeoka M Trifluoromethylation of Alkenes with Concomitant Introduction of Additional Functional Groups. Angew. Chem., Int. Ed 2014, 53, 8294–8308. - PubMed
- Merino E; Nevado C Addition of CF3 Across Unsaturated Moieties: A Powerful Functionalization Tool. Chem. Soc. Rev 2014, 43, 6598–6608. - PMC - PubMed
- Yin G; Mu X; Liu G Palladium (II)-Catalyzed Oxidative Difunctionalization of Alkenes: Bond Forming at a High-Valent Palladium Center. Acc. Chem. Res 2016, 49, 2413–2423. - PubMed
- Crossley SWM; Obradors C; Martinez RM; Shenvi RA Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins. Chem. Rev 2016, 116, 8912–9000. - PMC - PubMed
- Lan X-W; Wang N-X; Xing Y Recent Advances in Radical Difunctionalization of Simple Alkenes. Eur. J. Org. Chem 2017, 2017, 5821–5851.
- Dhungana RK; KC S; Basnet P; Giri R Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins. Chem. Rec 2018, 18, 1314–1340. - PubMed
- KC S; Giri R Strategies toward Dicarbofunctionalization of Unactivated Olefins by Combined Heck Carbometalation and Cross-Coupling. J. Org. Chem 2018, 83, 3013–3022. - PubMed
- Zhang J-S; Liu L; Chen T; Han LB Transition-Metal-Catalyzed Three-Component Difunctionalizations of Alkenes. Chem. -Asian J 2018, 13, 2277–2291. - PubMed
- Lin J; Song R-J; Hu M; Li J-H Recent Advances in the Intermolecular Oxidative Difunctionalization of Alkenes. Chem. Rec 2019, 19, 440–451. - PubMed
- Jiang H; Studer A Intermolecular Radical Carboamination of Alkenes. Chem. Soc. Rev 2020, 49, 1790–1811. - PubMed
- Li Y; Wu D; Cheng H; Yin G Difunctionalization of Alkenes Involving Metal Migration. Angew. Chem., Int. Ed 2020, 59, 7990–8003. - PubMed
-
-
- Beletskaya IP; Cheprakov AV The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev 2000, 100 (8), 3009–3066. - PubMed
- Dounay AB; Overman LE The Asymmetric Intramolecular Heck Reaction in Natural Product Total Synthesis. Chem. Rev 2003, 103, 2945–2963. - PubMed
- Mc Cartney D; Guiry PJ The Asymmetric Heck and Related Reactions. Chem. Soc. Rev 2011, 40, 5122–5150. - PubMed
-
- Trost BM; Van Vranken DL Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev 1996, 96, 395–422. - PubMed
- Trost BM Designing a Receptor for Molecular Recognition in a Catalytic Synthetic Reaction: Allylic Alkylation. Acc. Chem. Res 1996, 29, 355–364.
- Helmchen G Enantioselective Palladium-Catalyzed Allylic substitutions with Asymmetric chiral ligands. J. Organomet. Chem 1999, 576, 203–214.
- Trost BM; Crawley ML Asymmetric Transition-Metal-Catalyzed Allylic Alkylations: Applications in Total Synthesis. Chem. Rev 2003, 103, 2921–2944. - PubMed
- Trost BM; Machacek MR; Aponick A Predicting the Stereochemistry of Diphenylphosphino Benzoic Acid (DPPBA)-Based Palladium-Catalyzed Asymmetric Allylic Alkylation Reactions: A Working Model. Acc. Chem. Res 2006, 39, 747–760. - PubMed
- Lu Z; Ma S Metal-Catalyzed Enantioselective Allylation in Asymmetric Synthesis. Angew. Chem., Int. Ed 2008, 47, 258–297. - PubMed
- Trost BM Pd- and Mo-Catalyzed Asymmetric Allylic Alkylation. Org. Process Res. Dev 2012, 16, 185–194. - PMC - PubMed
-
- Schils D; Stappers F; Solberghe G; van Heck R; Coppens M; Van den Heuvel D; Van der Donck P; Callewaert T; Meeussen F; De Bie E; Eersels K; Schouteden E Ligandless Heck Coupling between a Halogenated Aniline and Acrylonitrile Catalyzed by Pd/C: Development and Optimization of an Industrial-Scale Heck Process for the Production of a Pharmaceutical Intermediate. Org. Process Res. Dev 2008, 12, 530–536.
- Applications of Transition Metal Catalysis in Drug Discovery and Development: An Industrial Perspective; Crawley ML, Trost BM, Eds.; Wiley: Hoboken, NJ, 2012.
Grants and funding
LinkOut - more resources
Full Text Sources
