Genome-editing approaches and applications: a brief review on CRISPR technology and its role in cancer
- PMID: 33732568
- PMCID: PMC7910401
- DOI: 10.1007/s13205-021-02680-4
Genome-editing approaches and applications: a brief review on CRISPR technology and its role in cancer
Abstract
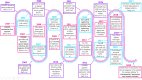
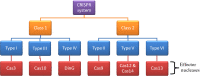
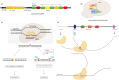
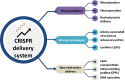
The development of genome-editing technologies in 1970s has discerned a new beginning in the field of science. Out of different genome-editing approaches such as Zing-finger nucleases, TALENs, and meganucleases, clustered regularly interspaced short palindromic repeats-CRISPR-associated protein 9 (CRISPR/Cas9) is a recent and versatile technology that has the ability of making changes to the genome of different organisms with high specificity. Cancer is a complex process that is characterized by multiple genetic and epigenetic changes resulting in abnormal cell growth and proliferation. As cancer is one of the leading causes of deaths worldwide, a large number of studies are done to understand the molecular mechanisms underlying the development of cancer. Because of its high efficiency and specificity, CRISPR/Cas9 has emerged as a novel and powerful tool in the field of cancer research. CRISPR/Cas9 has the potential to accelerate cancer research by dissecting tumorigenesis process, generating animal and cellular models, and identify drug targets for chemotherapeutic approaches. However, despite having tremendous potential, there are certain challenges associated with CRISPR/Cas9 such as safe delivery to the target, potential off-target effects and its efficacy which needs to be addressed prior to its clinical application. In this review, we give a gist of different genome-editing technologies with a special focus on CRISPR/Cas9 development, its mechanism of action and its applications, especially in different type of cancers. We also highlight the importance of CRISPR/Cas9 in generating animal models of different cancers. Finally, we present an overview of the clinical trials and discuss the challenges associated with translating CRISPR/Cas9 in clinical use.
Keywords: Animal models; CRISPR/Cas9; Cancer; Genetics; Genome editing; Nucleic acids.
© King Abdulaziz City for Science and Technology 2021.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

