Use of the Selective Cytopheretic Device in Critically Ill Children
- PMID: 33732992
- PMCID: PMC7938071
- DOI: 10.1016/j.ekir.2020.12.010
Use of the Selective Cytopheretic Device in Critically Ill Children
Abstract
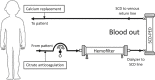
Introduction: Critically ill children with acute kidney injury (AKI) requiring continuous kidney replacement therapy (CKRT) are at increased risk of death. The selective cytopheretic device (SCD) promotes an immunomodulatory effect when circuit ionized calcium (iCa2+) is maintained at <0.40 mmol/l with regional citrate anticoagulation (RCA). In a randomized trial of adult patients on CRRT, those treated with the SCD maintaining an iCa2+ <0.40 mmol/l had improved survival/dialysis independence. We conducted a US Food and Drug Administration (FDA)-sponsored study to evaluate safety and feasibility of the SCD in 16 critically ill children.
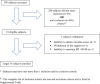
Methods: Four pediatric intensive care units (ICUs) enrolled children with AKI and multiorgan dysfunction receiving CKRT to receive the SCD integrated post-CKRT membrane. RCA was used to achieve a circuit iCa2+ level <0.40 mmol/l. Subjects received SCD treatment for 7 days or CKRT discontinuation, whichever came first.
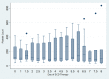
Results: The FDA target enrollment of 16 subjects completed the study from December 2016 to February 2020. Mean age was 12.3 ± 5.1 years, weight was 53.8 ± 28.9 kg, and median Pediatric Risk of Mortality II was 7 (range 2-19). Circuit iCa2+ levels were maintained at <0.40 mmol/l for 90.2% of the SCD therapy time. Median SCD duration was 6 days. Fifteen subjects survived SCD therapy; 12 survived to ICU discharge. All ICU survivors were dialysis independent at 60 days. No SCD-related adverse events (AEs) were reported.
Conclusion: Our data demonstrate that SCD therapy is feasible and safe in children who require CKRT. Although we cannot make efficacy claims, the 75% survival rate and 100% renal recovery rate observed suggest a possible favorable benefit-to-risk ratio.
Keywords: acute kidney injury; children; continuous kidney replacement therapy; selective cytopheretic device.
© 2020 International Society of Nephrology. Published by Elsevier Inc.
Figures

References
-
- Humes H.D., Fissell W.H., Weitzel W.F. The bioartificial kidney in the treatment of acute renal failure. Kidney Int Suppl. 2002;80:121–125. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

