Proteomimetic Zinc Finger Domains with Modified Metal-binding β-Turns
- PMID: 33733039
- PMCID: PMC7958900
- DOI: 10.1002/pep2.24177
Proteomimetic Zinc Finger Domains with Modified Metal-binding β-Turns
Abstract
The mimicry of protein tertiary folds by chains artificial in backbone chemical composition leads to proteomimetic analogues with potential utility as bioactive agents and as tools to shed light on biomacromolecule behavior. Notable successes toward such molecules have been achieved; however, as protein structural diversity is vast, design principles must be continually honed as they are applied to new prototype folding patterns. One specific structure where a gap remains in understanding how to effectively generate modified backbone analogues is the metal-binding β-turn found in zinc finger domains. Literature precedent suggests several factors that may act in concert, including the artificial moiety used to modify the turn, the sequence in which it is applied, and modifications present elsewhere in the domain. Here, we report efforts to gain insights into these issues and leverage these insights to construct a zinc finger mimetic with backbone modifications throughout its constituent secondary structures. We first conduct a systematic comparison of four turn mimetics in a common host sequence, quantifying relative efficacy for use in a metal-binding context. We go on to construct a proteomimetic zinc finger domain in which the helix, strands, and turn are simultaneously modified, resulting in a variant with 23% artificial residues, a tertiary fold indistinguishable from the prototype, and a folded stability comparable to the natural backbone on which the variant is based. Collectively, the results reported provide new insights into the effects of backbone modification on structure and stability of metal-binding domains and help inform the design of metalloprotein mimetics.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures

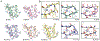
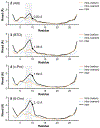

Similar articles
-
Heterogeneous-Backbone Proteomimetic Analogues of Lasiocepsin, a Disulfide-Rich Antimicrobial Peptide with a Compact Tertiary Fold.ACS Chem Biol. 2022 Apr 15;17(4):987-997. doi: 10.1021/acschembio.2c00138. Epub 2022 Mar 15. ACS Chem Biol. 2022. PMID: 35290019 Free PMC article.
-
Foldamer Tertiary Structure through Sequence-Guided Protein Backbone Alteration.Acc Chem Res. 2018 May 15;51(5):1220-1228. doi: 10.1021/acs.accounts.8b00048. Epub 2018 Apr 19. Acc Chem Res. 2018. PMID: 29672021 Free PMC article.
-
Protein Backbone Alteration in Non-Hairpin β-Turns: Impacts on Tertiary Folded Structure and Folded Stability.Chembiochem. 2023 Jun 1;24(11):e202300113. doi: 10.1002/cbic.202300113. Epub 2023 Mar 30. Chembiochem. 2023. PMID: 36920327 Free PMC article.
-
Analysis of folded structure and folding thermodynamics in heterogeneous-backbone proteomimetics.Methods Enzymol. 2021;656:93-122. doi: 10.1016/bs.mie.2021.04.009. Epub 2021 May 3. Methods Enzymol. 2021. PMID: 34325801 Free PMC article. Review.
-
Proteomimetics as protein-inspired scaffolds with defined tertiary folding patterns.Nat Chem. 2020 Apr;12(4):331-337. doi: 10.1038/s41557-020-0420-9. Epub 2020 Feb 6. Nat Chem. 2020. PMID: 32029906 Free PMC article. Review.
Cited by
-
Effects of altered backbone composition on the folding kinetics and mechanism of an ultrafast-folding protein.Chem Sci. 2023 Dec 4;15(2):675-682. doi: 10.1039/d3sc03976e. eCollection 2024 Jan 3. Chem Sci. 2023. PMID: 38179541 Free PMC article.
-
Studying Peptide-Metal Ion Complex Structures by Solution-State NMR.Int J Mol Sci. 2022 Dec 15;23(24):15957. doi: 10.3390/ijms232415957. Int J Mol Sci. 2022. PMID: 36555599 Free PMC article. Review.
-
Metal-Binding Foldamers.Chempluschem. 2021 Jan;86(1):137-145. doi: 10.1002/cplu.202000730. Chempluschem. 2021. PMID: 33415826 Free PMC article. Review.
-
Zinc as a Mediator Through the ROCK1 Pathway of Cognitive Impairment in Aluminum-Exposed Workers: A Clinical and Animal Study.Biol Trace Elem Res. 2024 Dec;202(12):5413-5428. doi: 10.1007/s12011-024-04119-2. Epub 2024 Feb 26. Biol Trace Elem Res. 2024. PMID: 38407795
-
Synthesis and Biological Activities of Some Metal Complexes of Peptides: A Review.BioTech (Basel). 2024 Apr 8;13(2):9. doi: 10.3390/biotech13020009. BioTech (Basel). 2024. PMID: 38651489 Free PMC article. Review.
References
-
- Gante J, Angew. Chem. Int. Ed 1994, 33, 1699–1720.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
