Pharmaceutically relevant (hetero)cyclic compounds and natural products from lignin-derived monomers: Present and perspectives
- PMID: 33733071
- PMCID: PMC7941040
- DOI: 10.1016/j.isci.2021.102211
Pharmaceutically relevant (hetero)cyclic compounds and natural products from lignin-derived monomers: Present and perspectives
Abstract
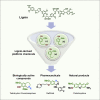
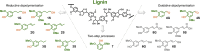
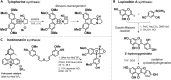
Lignin, the richest source of renewable aromatics on the planet, is an intriguing raw material for the construction of value-added aromatics. In the past decade, much progress has been made regarding the development of efficient lignin depolymerization methods, able to produce specific monophenol derivatives in high-enough selectivity and yields. This now serves as an excellent basis for developing powerful downstream conversion strategies toward a wide range of products, including fine chemical building blocks. The inherent structural features of lignin-derived platform chemicals undoubtedly inspire the development of novel, creative, atom-economic synthetic routes toward biologically active molecules or natural products. In this perspective we attempt to bridge the structural features of lignin-derived platform chemicals with existing synthetic strategies toward the construction of heterocycles and provide a summary of efforts for the production of natural products from aromatics that can be, in principle, obtained from lignin. Last, we comment on the latest efforts that present entire value-chains from wood to valuable pharmaceutically relevant compounds.
Keywords: Chemistry; Green Chemistry; Organic Chemistry; Process Biotechnology.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Abu-Omar M.M., Barta K., Beckham G.T., Luterbacher J.S., Ralph J., Rinaldi R., Román-Leshkov Y., Samec J.S.M., Sels B.F., Wang F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2020 doi: 10.1039/d0ee02870c. - DOI
-
- Ackermann L., Althammer A. Domino N-H/C-H bond activation: palladium-catalyzed synthesis of annulated heterocycles using dichloro(hetero)arenes. Angew. Chem. Int. Ed. 2007;46:1627–1629. - PubMed
-
- Afanasenko A., Barta K. Taking the ‘high road’ to renewable beta-amino acids. 2019. https://chemistrycommunity.nature.com/posts/56323-taking-the-high-road-t...
-
- Alsarraf J., Bilodeau J.-F., Legault J., Simard F., Pichette A. Exploring the biomass-derived chemical space emerging from natural dihydrochalcones through the single-step hemisynthesis of antibacterial balsacones. ACS Sustain. Chem. Eng. 2020;8:6194–6199.
-
- Bariwal J., Van Der Eycken E. C-N bond forming cross-coupling reactions: an overview. Chem. Soc. Rev. 2013;42:9283–9303. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

