A hepatitis B virus transgenic mouse model with a conditional, recombinant, episomal genome
- PMID: 33733079
- PMCID: PMC7940981
- DOI: 10.1016/j.jhepr.2021.100252
A hepatitis B virus transgenic mouse model with a conditional, recombinant, episomal genome
Abstract
Background & aims: Development of new and more effective therapies against hepatitis B virus (HBV) is limited by the lack of suitable small animal models. The HBV transgenic mouse model containing an integrated overlength 1.3-mer construct has yielded crucial insights, but this model unfortunately lacks covalently closed circular DNA (cccDNA), the episomal HBV transcriptional template, and cannot be cured given that HBV is integrated in every cell.
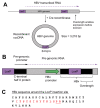
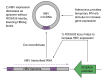
Methods: To solve these 2 problems, we generated a novel transgenic mouse (HBV1.1X), which generates an excisable circular HBV genome using Cre/LoxP technology. This model possesses a HBV1.1-mer cassette knocked into the ROSA26 locus and is designed for stable expression of viral proteins from birth, like the current HBV transgenic mouse model, before genomic excision with the introduction of Cre recombinase.
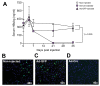
Results: We demonstrated induction of recombinant cccDNA (rcccDNA) formation via viral or transgenic Cre expression in HBV1.1X mice, and the ability to regulate HBsAg and HBc expression with Cre in mice. Tamoxifen-inducible Cre could markedly downregulate baseline HBsAg levels from the integrated HBV genome. To demonstrate clearance of HBV from HBV1.1X mice, we administered adenovirus expressing Cre, which permanently and significantly reduced HBsAg and core antigen levels in the murine liver via rcccDNA excision and a subsequent immune response.
Conclusions: The HBV1.1X model is the first Cre-regulatable HBV transgenic mouse model and should be of value to mimic chronic HBV infection, with neonatal expression and tolerance of HBV antigens, and on-demand modulation of HBV expression.
Lay summary: Hepatitis B virus (HBV) can only naturally infect humans and chimpanzees. Mouse models have been developed with the HBV genome integrated into mouse chromosomes, but this prevents mice from being cured. We developed a new transgenic mouse model that allows for HBV to be excised from mouse chromosomes to form a recombinant circular DNA molecule resembling the natural circular HBV genome. HBV expression could be reduced in these mice, enabling curative therapies to be tested in this new mouse model.
Keywords: AAV, adeno-associated virus; Ad, adenovirus; Cre/LoxP; HBx, HBV X-protein; Hepatitis B virus; ORF, open reading frame; PFUs, plaque-forming units; Transgenic mouse; cccDNA; cccDNA, covalently closed circular DNA; pgRNA, pregenomic RNA; rcccDNA, recombinant cccDNA.
© 2021 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures




References
-
- Schweitzer A., Horn J., Mikolajczyk R.T., Krause G., Ott J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. - PubMed
-
- Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972–1984. - PubMed
-
- Reaiche G.Y., Le Mire M.F., Mason W.S., Jilbert A.R. The persistence in the liver of residual duck hepatitis B virus covalently closed circular DNA is not dependent upon new viral DNA synthesis. Virology. 2010;406:286–292. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

