Real-World Experience with Coformulated Ledipasvir and Sofosbuvir for HIV-Positive Patients with HCV Genotype 2 Infection: A Multicenter, Retrospective Study
- PMID: 33733316
- PMCID: PMC8116398
- DOI: 10.1007/s40121-021-00424-8
Real-World Experience with Coformulated Ledipasvir and Sofosbuvir for HIV-Positive Patients with HCV Genotype 2 Infection: A Multicenter, Retrospective Study
Abstract
Introduction: While coformulated ledipasvir (90 mg)/sofosbuvir (400 mg) (LDV/SOF) is approved for the treatment of hepatitis C virus (HCV) genotype 2 (GT2) infection in Taiwan, Japan, and New Zealand, data regarding its use for HIV (Human Immunodeficiency Virus)-positive patients infected with HCV GT2 are sparse. We aimed to assess the effectiveness and tolerability of LDV/SOF for HIV-positive patients with HCV GT2 coinfection.
Methods: From January 2019 to July 2020, consecutive HIV-positive Taiwanese patients infected with HCV GT2 who received LDV/SOF were retrospectively included for analysis. The effectiveness was determined by sustained virologic response 12 weeks off-therapy (SVR12).
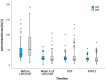
Results: Of the 114 patients (mean age, 38.6 years) initiating LDV/SOF during the study period, 0.9% had liver cirrhosis and 4.4% were HCV treatment-experienced. All patients had estimated glomerular filtration rate (eGFR) > 30 ml/min/1.73 m2 and were receiving antiretroviral therapy with 98.2% having CD4 counts ≥ 200 cells/mm3 and 93.9% plasma HIV RNA load < 50 copies/ml. Antiretrovirals prescribed included tenofovir alafenamide/emtricitabine in 42.1%, tenofovir disoproxil fumarate (TDF)/emtricitabine 18.4%, other nucleoside reverse transcriptase inhibitors (NRTIs) 39.5%, non-NRTIs 12.3%, protease inhibitors 13.2%, and integrase inhibitors 74.6%. All patients had undetectable plasma HCV RNA load at the end of treatment, and 96.5% achieved SVR12 in intention-to-treat analysis. The on-treatment eGFR decline was more pronounced in those receiving TDF-containing antiretroviral therapy (mean change, - 8.33 ml/min/1.73 m2), which was reversible after discontinuation of LDV/SOF. None of the patients interrupted LDV/SOF during the 12-week treatment course.
Conclusion: Similar to the response observed among HIV-negative patients, LDV/SOF is effective for HIV-positive patients coinfected with HCV GT2.
Keywords: Antiretroviral therapy; Direct-acting antivirals; End-of-treatment response; Estimated glomerular filtration rate; Ledipasvir; Sofosbuvir; Sustained virologic response.
Figures

Similar articles
-
Nephrotoxicity Associated with Concomitant Use of Ledipasvir-Sofosbuvir and Tenofovir in a Patient with Hepatitis C Virus and Human Immunodeficiency Virus Coinfection.Pharmacotherapy. 2016 Sep;36(9):e148-53. doi: 10.1002/phar.1803. Pharmacotherapy. 2016. PMID: 27459733
-
HIV/HCV therapy with ledipasvir/sofosbuvir after randomized switch to emtricitabine-tenofovir alafenamide-based single-tablet regimens.PLoS One. 2020 Jan 29;15(1):e0224875. doi: 10.1371/journal.pone.0224875. eCollection 2020. PLoS One. 2020. PMID: 31995556 Free PMC article. Clinical Trial.
-
Real-world effectiveness of direct-acting antivirals in people living with human immunodeficiency virus and hepatitis C virus genotype 6 infections.World J Gastroenterol. 2022 Mar 21;28(11):1172-1183. doi: 10.3748/wjg.v28.i11.1172. World J Gastroenterol. 2022. PMID: 35431505 Free PMC article.
-
Glomerular filtration rate change during chronic hepatitis C treatment with Sofosbuvir/Ledipasvir in HCV/HIV Coinfected patients treated with Tenofovir and a boosted protease inhibitor: an observational prospective study.BMC Infect Dis. 2018 Aug 3;18(1):364. doi: 10.1186/s12879-018-3278-3. BMC Infect Dis. 2018. PMID: 30075765 Free PMC article.
-
Ledipasvir/Sofosbuvir (Harvoni): For the Treatment of Chronic Hepatitis C Virus (CHC) G1 Infection in Adults [Internet].Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2015 Jul. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2015 Jul. PMID: 27227210 Free Books & Documents. Review.
Cited by
-
Taiwan accelerates its efforts to eliminate hepatitis C.Glob Health Med. 2021 Oct 31;3(5):293-300. doi: 10.35772/ghm.2021.01064. Glob Health Med. 2021. PMID: 34782872 Free PMC article. Review.
-
Performance of Hepatitis C Virus (HCV) Core Antigen Assay in the Diagnosis of Recently Acquired HCV Infection among High-Risk Populations.Microbiol Spectr. 2022 Jun 29;10(3):e0034522. doi: 10.1128/spectrum.00345-22. Epub 2022 May 17. Microbiol Spectr. 2022. PMID: 35579445 Free PMC article.
References
-
- World Health Organization. Global Hepatitis Report, 2017. Available at: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. Accessed 12 February 2021.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

