Toll-like receptor 4 is activated by platinum and contributes to cisplatin-induced ototoxicity
- PMID: 33733573
- PMCID: PMC8097357
- DOI: 10.15252/embr.202051280
Toll-like receptor 4 is activated by platinum and contributes to cisplatin-induced ototoxicity
Abstract
Toll-like receptor 4 (TLR4) recognizes bacterial lipopolysaccharide (LPS) and can also be activated by some Group 9/10 transition metals, which is believed to mediate immune hypersensitivity reactions. In this work, we test whether TLR4 can be activated by the Group 10 metal platinum and the platinum-based chemotherapeutic cisplatin. Cisplatin is invaluable in childhood cancer treatment but its use is limited due to a permanent hearing loss (cisplatin-induced ototoxicity, CIO) adverse effect. We demonstrate that platinum and cisplatin activate pathways downstream of TLR4 to a similar extent as the known TLR4 agonists LPS and nickel. We further show that TLR4 is required for cisplatin-induced inflammatory, oxidative, and cell death responses in hair cells in vitro and for hair cell damage in vivo. Finally, we identify a TLR4 small molecule inhibitor able to curtail cisplatin toxicity in vitro. Thus, our findings indicate that TLR4 is a promising therapeutic target to mitigate CIO.
Keywords: TLR4; chemotherapy; cisplatin; hearing loss; ototoxicity.
© 2021 The Authors. Published under the terms of the CC BY NC ND 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

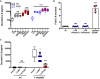
Activity of an NF‐κB reporter relative to vehicle control in human embryonic kidney cells that express TLR4 (hTLR4) or an isogenic control cell line that does not express TLR4 (null2) and were stimulated with vehicle (veh), 1 ng/ml LPS, 400 µM nickel chloride, or 25, 50, or 100 µM platinum(II) chloride, and platinum(IV) chloride (n = 3 independent biological replicates).
As per panel (A), secreted IL‐8 was monitored as a metric of TLR4 activation upon stimulation with 50 pg/ml LPS, 200 µM nickel chloride, 100 µM platinum(II) chloride, or 100 µM platinum(IV) chloride (n = 4 independent biological replicates).
IL‐8 secretion in HEK‐hTLR4 and HEK‐null2 cells following treatment with cisplatin at the indicated concentrations (n = 3 independent biological replicates).
IL‐8 secretion in HEK‐hTLR4 cells pre‐treated with 4 µ M TAK242 (TLR4 inhibitor) or vehicle, and subsequent treatment with 50 pg/ml LPS, 200 µM nickel chloride, or 25 µM cisplatin (n = 3 independent biological replicates). Mock cells were not subject to pre‐treatment prior to agonist addition.

Phospho‐signaling in HEI‐OC1 cells following treatment with 20 µM cisplatin or 10 ng/ml LPS at the indicated time points (representative of 3 independent biological replicates).
IRF3 reporter activity in HEI‐OC1 cells treated with and without 20 µM cisplatin after 24 h (n = 4 independent biological replicates).
IL‐8 secretion in HEK cells stably expressing hTLR4 but not MD‐2 (HEK‐isoTLR4), transfected with empty vector (EV) or MD‐2 and left untreated (nil) or treated with 1 ng/ml LPS, 200 µM nickel chloride, 2 µg/ml HMGB1, 100 µM platinum(II) chloride, 100 µM platinum(IV) chloride, or 25 µM cisplatin (n = 3 or 4 independent biological replicates).
Fold IL‐8 secreted (relative to nil treatment) in MD‐2‐deficient HeLa cells treated with 10 or 100 ng/ml LPS or 25 µM cisplatin (n = 4 independent biological replicates).
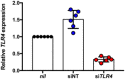
IL‐8 secretion in HeLa cells transfected with non‐targeting (siNT) or TLR4‐targeting (siTLR4) siRNA and left untreated (nil), or treated with 30 µM cisplatin (n = 3 independent biological replicates). Mock cells were not subject to siRNA treatment prior.
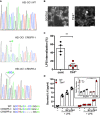
Comparison of genomic DNA at the Tlr4 locus from Tlr4 −/− HEI‐OC1 and wild‐type cells. Sequences from the Tlr4 −/− cell line contained a single nucleotide insertion or four nucleotide deletion and summarized below.
Anti‐TLR4 staining in Tlr4 −/− and control HEI‐OC1 cells. Bars (lower right) are 50 µm.
Flow cytometric analysis of conjugated LPS internalization in Tlr4 −/− and control HEI‐OC1 cells (n = 4 independent biological replicates).
IL‐6 secretion in Tlr4 −/− and control HEI‐OC1 cells transfected with empty vector (EV), Tlr4 (pTlr4), or left untransfected (−) and subsequently treated with 100 ng/ml LPS (n = 4 independent biological replicates). Inset, fold induction of IL‐6 secretion was determined relative to the untransfected cells treated with LPS.

- A–D
HEI‐OC1 cells containing a Tlr4 deletion (Tlr4 −/−) were compared to HEI‐OC1 non‐targeting (NT) control cells and assessed for cell viability (A), Annexin V/propidium iodide staining (B), ROS generation (C), and IL‐6 secretion (D) following cisplatin treatment at the indicated concentrations (n = 3 independent biological replicates).
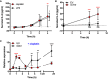
IL‐6 secretion in HEI‐OC1 cells treated with 100 pg/ml LPS or 20 µM cisplatin (n = 3 or 4 independent biological replicates).
IL‐6 secretion in TLR4−/− cells transfected with empty vector (EV) or mouse Tlr4 (mTlr4) following treatment with 20 µM cisplatin (n = 6 independent biological replicates).
Il6 and Tlr4 transcript levels in HEI‐OC1 cells following treatment with 20 µM cisplatin for the indicated times (n = 3 independent biological replicates).
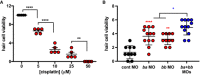
Hair cell viability in larval zebrafish (assessed by DASPEI staining) following cisplatin treatment at the indicated concentrations.
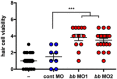
Hair cell viability in larval zebrafish pre‐treated with control‐, tlr4ba‐, and/or tlr4bb‐targeting morpholino oligonucleotides (MO) and subsequently treated with 15 µM cisplatin.
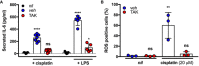
IL‐6 secretion in HEI‐OC1 cells pre‐treated with DMF vehicle (veh), 4 µM TAK242 (TAK), or left untreated (nil) following treatment with 10 ng/ml LPS or 20 µM cisplatin (n = 5–7 independent biological replicates).
ROS generation in HEI‐OC1 cells pre‐treated with DMF vehicle (veh), 4 µM TAK242 (TAK), or left untreated (nil) following treatment with 20 µM cisplatin (n = 3 independent biological replicates).
References
-
- American Childhood Cancer Organization (2017) US Childhood Cancer Statistics
-
- Blakley BW, Myers SF (1993) Patterns of hearing loss resulting from cis‐platinum therapy. Otolaryngol Head Neck Surg 109: 385–391 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

