Poly(Glycerol Sebacate) in Biomedical Applications-A Review of the Recent Literature
- PMID: 33733604
- PMCID: PMC11468981
- DOI: 10.1002/adhm.202002026
Poly(Glycerol Sebacate) in Biomedical Applications-A Review of the Recent Literature
Abstract
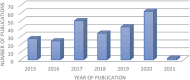
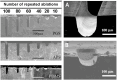
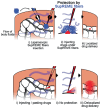
Poly(glycerol sebacate) (PGS) continues to attract attention for biomedical applications owing to its favorable combination of properties. Conventionally polymerized by a two-step polycondensation of glycerol and sebacic acid, variations of synthesis parameters, reactant concentrations or by specific chemical modifications, PGS materials can be obtained exhibiting a wide range of physicochemical, mechanical, and morphological properties for a variety of applications. PGS has been extensively used in tissue engineering (TE) of cardiovascular, nerve, cartilage, bone and corneal tissues. Applications of PGS based materials in drug delivery systems and wound healing are also well documented. Research and development in the field of PGS continue to progress, involving mainly the synthesis of modified structures using copolymers, hybrid, and composite materials. Moreover, the production of self-healing and electroactive materials has been introduced recently. After almost 20 years of research on PGS, previous publications have outlined its synthesis, modification, properties, and biomedical applications, however, a review paper covering the most recent developments in the field is lacking. The present review thus covers comprehensively literature of the last five years on PGS-based biomaterials and devices focusing on advanced modifications of PGS for applications in medicine and highlighting notable advances of PGS based systems in TE and drug delivery.
Keywords: biodegradable polyester; bioelectronics; biomedical applications; composites; poly(glycerol sebacate); tissue engineering.
© 2021 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Gadomska‐Gajadhur A., Wrzecionek M., Matyszczak G., Piętowski P., Więcław M., Ruśkowski P., Org. Process Res. Dev. 2018, 22, 1793.
-
- Wang Y., Ameer G. A., Sheppard B. J., Langer R., Nat. Biotechnol. 2002, 20, 602. - PubMed
-
- Rai R., Tallawi M., Grigore A., Boccaccini A. R., Prog. Polym. Sci. 2012, 37, 1051.
-
- Matyszczak G., Wrzecionek M., Gadomska‐Gajadhur A., Ruśkowski P., Org. Process Res. Dev. 2020, 24, 1104.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

