DHA intake relates to better cerebrovascular and neurodegeneration neuroimaging phenotypes in middle-aged adults at increased genetic risk of Alzheimer disease
- PMID: 33733657
- PMCID: PMC8168359
- DOI: 10.1093/ajcn/nqab016
DHA intake relates to better cerebrovascular and neurodegeneration neuroimaging phenotypes in middle-aged adults at increased genetic risk of Alzheimer disease
Abstract
Background: The number of APOE-ε4 alleles is a major nonmodifiable risk factor for sporadic Alzheimer disease (AD). There is increasing evidence on the benefits of dietary DHA (22:6n-3) before the onset of AD symptoms, particularly in APOE-ε4 carriers. Brain alterations in the preclinical stage can be detected by structural MRI.
Objectives: We aimed, in middle-aged cognitively unimpaired individuals at increased risk of AD, to cross-sectionally investigate whether dietary DHA intake relates to cognitive performance and to MRI-based markers of cerebral small vessel disease and AD-related neurodegeneration, exploring the effect modification by APOE-ε4 status.
Methods: In 340 participants of the ALFA (ALzheimer and FAmilies) study, which is enriched for APOE-ε4 carriership (n = 122, noncarriers; n = 157, 1 allele; n = 61, 2 alleles), we assessed self-reported DHA intake through an FFQ. We measured cognitive performance by administering episodic memory and executive function tests. We performed high-resolution structural MRI to assess cerebral small vessel disease [white matter hyperintensities (WMHs) and cerebral microbleeds (CMBs)] and AD-related brain atrophy (cortical thickness in an AD signature). We constructed regression models adjusted for potential confounders, exploring the interaction DHA × APOE-ε4.
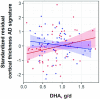
Results: We observed no significant associations between DHA and cognitive performance or WMH burden. We observed a nonsignificant inverse association between DHA and prevalence of lobar CMBs (OR: 0.446; 95% CI: 0.195, 1.018; P = 0.055). DHA was found to be significantly related to greater cortical thickness in the AD signature in homozygotes but not in nonhomozygotes (P-interaction = 0.045). The association strengthened when analyzing homozygotes and nonhomozygotes matched for risk factors.
Conclusions: In cognitively unimpaired APOE-ε4 homozygotes, dietary DHA intake related to structural patterns that may result in greater resilience to AD pathology. This is consistent with the current hypothesis that those subjects at highest risk would obtain the largest benefits from DHA supplementation in the preclinical stage.This trial was registered at clinicaltrials.gov as NCT01835717.
Keywords: brain atrophy; cerebral small vessel disease; cognition; markers; omega-3 fatty acids; white matter hyperintensities.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Society for Nutrition.
Figures
Comment in
-
Pushing the boundaries of precision nutrition to tackle Alzheimer's disease: is there a role for DHA?Am J Clin Nutr. 2021 Jun 1;113(6):1396-1397. doi: 10.1093/ajcn/nqab085. Am J Clin Nutr. 2021. PMID: 33851196 No abstract available.
References
-
- Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653–6. - PubMed
-
- Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17:1006–15. - PubMed
-
- Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr. 2015;103:330–40. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

