Leveraging Marine Natural Products as a Platform to Tackle Bacterial Resistance and Persistence
- PMID: 33733746
- PMCID: PMC8428769
- DOI: 10.1021/acs.accounts.1c00007
Leveraging Marine Natural Products as a Platform to Tackle Bacterial Resistance and Persistence
Abstract
Antimicrobial resistance to existing antibiotics represents one of the greatest threats to human health and is growing at an alarming rate. To further complicate treatment of bacterial infections, many chronic infections are the result of bacterial biofilms that are tolerant to treatment with antibiotics because of the presence of metabolically dormant persister cell populations. Together these threats are creating an increasing burden on the healthcare system, and a "preantibiotic" age is on the horizon if significant action is not taken by the scientific and medical communities. While the golden era of antibiotic discovery (1940s-1960s) produced most of the antibiotic classes in clinical use today, followed by several decades of limited development, there has been a resurgence in antibiotic drug discovery in recent years fueled by the academic and biotech sectors. Historically, great success has been achieved by developing next-generation variants of existing classes of antibiotics, but there remains a dire need for the identification of novel scaffolds and/or antimicrobial targets to drive future efforts to overcome resistance and tolerance. In this regard, there has been no more valuable source for the identification of antibiotics than natural products, with 69-77% of approved antibiotics either being such compounds or being derived from them.Our group has developed a program centered on the chemical synthesis and chemical microbiology of marine natural products with unusual structures and promising levels of activity against multidrug-resistant (MDR) bacterial pathogens. As we are motivated by preparing and studying the biological effects of these molecules, we are not initially pursuing a biological question but instead are allowing the observed phenotypes and activities to guide the ultimate project direction. In this Account, our recent efforts on the synoxazolidinone, lipoxazolidinone, and batzelladine natural products will be discussed and placed in the context of the field's greatest challenges and opportunities. Specifically, the synoxazolidinone family of 4-oxazolidinone-containing natural products has led to the development of several chemical methods to prepare antimicrobial scaffolds and has revealed compounds with potent activity as adjuvants to treat bacterial biofilms. Bearing the same 4-oxazolidinone core, the lipoxazolidinones have proven to be potent single-agent antibiotics. Finally, our synthetic efforts toward the batzelladines revealed analogues with activity against a number of MDR pathogens, highlighted by non-natural stereochemical isomers with superior activity and simplified synthetic access. Taken together, these studies provide several distinct platforms for the development of novel therapeutics that can add to our arsenal of scaffolds for preclinical development and can provide insight into the biochemical processes and pathways that can be targeted by small molecules in the fight against antimicrobial-resistant and -tolerant infections. We hope that this work will serve as inspiration for increased efforts by the scientific community to leverage synthetic chemistry and chemical microbiology toward novel antibiotics that can combat the growing crisis of MDR and tolerant bacterial infections.
Conflict of interest statement
The authors declare the following competing financial interest(s): J.G.P. is the founder of Synoxa Sciences, Inc., an early-stage biotechnology company focused on developing novel antibiotics with efficacy against bacterial biofilms.
Figures
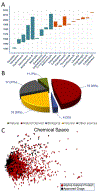
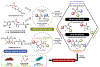


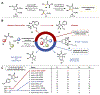




References
-
- Shymanska NV; An IH; Pierce JG A Rapid Synthesis of 4-Oxazolidinones: Total Synthesis of Synoxazolidinones A and B. Angew. Chem., Int. Ed 2014, 53 (21), 5401–5404. - PubMed
-
This paper reports heterocycle methods development, the initial synthesis of synoxazolidinone A and B, and antimicrobial evaluation against ESKAPE pathogens.
-
- Mills JJ; Robinson KR; Zehnder TE; Pierce JG Synthesis and Biological Evaluation of the Antimicrobial Natural Product Lipoxazolidinone A. Angew. Chem., Int. Ed 2018, 57 (28), 8682–8686. - PMC - PubMed
-
This report describes heterocycle methods development, the initial synthesis of lipoxazolidinone A, and antimicrobial evaluation against ESKAPE pathogens.
-
- Frohock BH; Gilbertie JM; Daiker JC; Schnabel LV; Pierce JG 5-Benzylidene-4-Oxazolidinones Are Synergistic with Antibiotics for the Treatment of Staphylococcus aureus Biofilms. ChemBioChem 2020, 21 (7), 933–937. - PMC - PubMed
-
This paper reports the optimization of the synoxazolidinone scaffold and the demonstration of synergy with existing antibiotics to treat MDR Staphylococcus aureus biofilms.
-
- Lin Y-C; Ribaucourt A; Moazami Y; Pierce JG Concise Synthesis and Antimicrobial Evaluation of the Guanidinium Alkaloid Batzelladine D: Development of a Stereodivergent Strategy. J. Am. Chem. Soc 2020, 142 (21), 9850–9857. - PMC - PubMed
-
This article describes the first antimicrobial evaluation of a series of stereochemical analogues of batzelladine D. A key bicyclic β-lactam intermediate allows a stereodivergent route and a streamlined synthesis.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

