Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study
- PMID: 33734013
- PMCID: PMC8023607
- DOI: 10.1080/22221751.2021.1905488
Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study
Abstract
Seasonal human coronaviruses (HCoVs) including HCoV-229E, -OC43, -NL63, and -HKU1 widely spread in global human populations. However, the relevance of humoral response against seasonal HCoVs to COVID-19 pathogenesis is elusive. In this study, we profiled the temporal changes of IgG antibody against spike proteins (S-IgG) of SARS-CoV-2 and seasonal HCoVs in 838 plasma samples collected from 344 COVID-19 patients. We tested the antigenic cross-reactivities of S protein between SARS-CoV-2 and seasonal HCoVs and evaluated the correlations between the levels of HCoV-OC43 S-IgG and the disease severity in COVID-19 patients. We found that SARS-CoV-2 S-IgG titres mounted until days 22-28, whereas HCoV-OC43 antibody titres increased until days 15-21 and then plateaued until day 46. However, IgG titres against HCoV-NL63, -229E, and -HKU1 showed no significant increase. A two-way cross-reactivity was identified between SARS-CoV-2 and HCoV-OC43. Neutralizing antibodies against SARS-CoV-2 were not detectable in healthy controls who were positive for HCoV-OC43 S-IgG. HCoV-OC43 S-IgG titres were significantly higher in patients with severe disease than those in mild patients at days 1-21 post symptom onset (PSO). Higher levels of HCoV-OC43 S-IgG were also observed in patients requiring mechanical ventilation. At days 1-10 PSO, HCoV-OC43 S-IgG titres correlated to disease severity in the age group over 60. Our data indicate that there is a correlation between cross-reactive antibody against HCoV-OC43 spike protein and disease severity in COVID-19 patients.
Keywords: Cross-reactivity; HCoV-OC43; SARS-CoV-2; antibody; disease severity.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


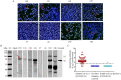

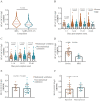
References
-
- Long QX, Liu BZ, Deng HJ, et al. . Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
