Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial
- PMID: 33734299
- PMCID: PMC7974835
- DOI: 10.1001/jama.2021.4152
Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial
Abstract
Importance: Thrombotic events are commonly reported in critically ill patients with COVID-19. Limited data exist to guide the intensity of antithrombotic prophylaxis.
Objective: To evaluate the effects of intermediate-dose vs standard-dose prophylactic anticoagulation among patients with COVID-19 admitted to the intensive care unit (ICU).
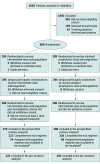
Design, setting, and participants: Multicenter randomized trial with a 2 × 2 factorial design performed in 10 academic centers in Iran comparing intermediate-dose vs standard-dose prophylactic anticoagulation (first hypothesis) and statin therapy vs matching placebo (second hypothesis; not reported in this article) among adult patients admitted to the ICU with COVID-19. Patients were recruited between July 29, 2020, and November 19, 2020. The final follow-up date for the 30-day primary outcome was December 19, 2020.
Interventions: Intermediate-dose (enoxaparin, 1 mg/kg daily) (n = 276) vs standard prophylactic anticoagulation (enoxaparin, 40 mg daily) (n = 286), with modification according to body weight and creatinine clearance. The assigned treatments were planned to be continued until completion of 30-day follow-up.
Main outcomes and measures: The primary efficacy outcome was a composite of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days, assessed in randomized patients who met the eligibility criteria and received at least 1 dose of the assigned treatment. Prespecified safety outcomes included major bleeding according to the Bleeding Academic Research Consortium (type 3 or 5 definition), powered for noninferiority (a noninferiority margin of 1.8 based on odds ratio), and severe thrombocytopenia (platelet count <20 ×103/µL). All outcomes were blindly adjudicated.
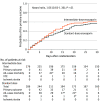
Results: Among 600 randomized patients, 562 (93.7%) were included in the primary analysis (median [interquartile range] age, 62 [50-71] years; 237 [42.2%] women). The primary efficacy outcome occurred in 126 patients (45.7%) in the intermediate-dose group and 126 patients (44.1%) in the standard-dose prophylaxis group (absolute risk difference, 1.5% [95% CI, -6.6% to 9.8%]; odds ratio, 1.06 [95% CI, 0.76-1.48]; P = .70). Major bleeding occurred in 7 patients (2.5%) in the intermediate-dose group and 4 patients (1.4%) in the standard-dose prophylaxis group (risk difference, 1.1% [1-sided 97.5% CI, -∞ to 3.4%]; odds ratio, 1.83 [1-sided 97.5% CI, 0.00-5.93]), not meeting the noninferiority criteria (P for noninferiority >.99). Severe thrombocytopenia occurred only in patients assigned to the intermediate-dose group (6 vs 0 patients; risk difference, 2.2% [95% CI, 0.4%-3.8%]; P = .01).
Conclusions and relevance: Among patients admitted to the ICU with COVID-19, intermediate-dose prophylactic anticoagulation, compared with standard-dose prophylactic anticoagulation, did not result in a significant difference in the primary outcome of a composite of adjudicated venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days. These results do not support the routine empirical use of intermediate-dose prophylactic anticoagulation in unselected patients admitted to the ICU with COVID-19.
Trial registration: ClinicalTrials.gov Identifier: NCT04486508.
Conflict of interest statement
Figures

Comment in
-
Finding the Optimal Thromboprophylaxis Dose in Patients With COVID-19.JAMA. 2021 Apr 27;325(16):1613-1615. doi: 10.1001/jama.2021.4295. JAMA. 2021. PMID: 33734290 No abstract available.
-
When to use anticoagulation in COVID-19.Thromb Res. 2021 Aug;204:136-137. doi: 10.1016/j.thromres.2021.06.005. Epub 2021 Jun 16. Thromb Res. 2021. PMID: 34176588 Free PMC article. No abstract available.
References
-
- Bikdeli B, Madhavan MV, Jimenez D, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function . COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: jacc state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950-2973. doi: 10.1016/j.jacc.2020.04.031 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

