Intravitreal Aflibercept Injection vs Sham as Prophylaxis Against Conversion to Exudative Age-Related Macular Degeneration in High-risk Eyes: A Randomized Clinical Trial
- PMID: 33734306
- PMCID: PMC7974836
- DOI: 10.1001/jamaophthalmol.2021.0221
Intravitreal Aflibercept Injection vs Sham as Prophylaxis Against Conversion to Exudative Age-Related Macular Degeneration in High-risk Eyes: A Randomized Clinical Trial
Abstract
Importance: Anti-vascular endothelial growth factor (VEGF) agents may provide a prophylactic effect in high-risk eyes with intermediate dry age-related macular degeneration (AMD) against conversion to exudative AMD (eAMD), lowering the risk of vision loss.
Objective: To evaluate intravitreal aflibercept injection (IAI) as prophylaxis against the conversion to eAMD in high-risk eyes at 24 months.
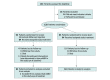
Design, setting, and participants: This single-masked, sham-controlled, randomized clinical trial performed at 4 US clinical sites enrolled patients with intermediate AMD in 1 eye (study eye), defined as presence of more than 10 medium drusen (≥63 to <125 μm), at least 1 large druse (≥125 μm), and/or retinal pigmentary changes, and eAMD in the fellow eye. Patients were treated from June 23, 2015, to March 13, 2019.
Interventions: Intravitreal aflibercept injection (2 mg) or sham quarterly injection for 24 months (1:1 randomization).
Main outcomes and measures: The primary end point was the proportion of patients with conversion to eAMD at month 24 characterized by development of choroidal neovascularization, as assessed by leakage on fluorescein angiography and fluid on spectral-domain optical coherence tomography by an independent masked reading center.
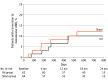
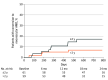
Results: Of 128 patients enrolled, 127 (63 in the IAI group and 64 in the sham group) were included in the primary analysis (68 men [53.5%]; mean [SD] age, 76.5 [8.1] years). Baseline demographic and clinical characteristics were balanced between the groups. By month 24, 6 patients (9.5%) in the IAI group and 7 (10.9%) in the sham group developed eAMD (P = .98). Patients with a history of eAMD for longer than 2 years in their fellow eye at baseline showed a lower rate of conversion to eAMD in the study eye compared with those with a history of eAMD for 2 years or less in the fellow eye. Safety was consistent with previous studies involving intravitreal anti-VEGF injections.
Conclusions and relevance: In this evaluation of quarterly anti-VEGF exposure as prophylaxis to reduce conversion of eyes with high-risk dry AMD to eAMD, the rates of conversion were not lower in the IAI group compared with the sham treatment group at month 24. Understanding the mechanism of conversion to eAMD and therapies that could prevent this event remains an important unmet need.
Trial registration: ClinicalTrials.gov Identifier: NCT02462889.
Conflict of interest statement
Figures
References
-
- Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL III; Age-Related Eye Disease Study Research Group . Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS): AREDS report No. 19. Ophthalmology. 2005;112(4):533-539. doi:10.1016/j.ophtha.2004.10.047 - DOI - PMC - PubMed
-
- Age-Related Eye Disease Study Research Group . A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report No. 8. Arch Ophthalmol. 2001;119(10):1417-1436. doi:10.1001/archopht.119.10.1417 - DOI - PMC - PubMed
-
- Lujan BJ ZL, Hopkins JJ. Comparison of spectral domain (SD-OCT) and fluorescein angiography (FA) conversion rates in fellow eyes of HARBOR patients. Presented at: 2012 Joint Meeting of the American Academy of Ophthalmology; November 10-13, 2012; Chicago, IL.
-
- Maguire MG, Daniel E, Shah AR, et al. ; Comparison of Age-Related Macular Degeneration Treatments Trials (CATT Research Group) . Incidence of choroidal neovascularization in the fellow eye in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120(10):2035-2041. doi:10.1016/j.ophtha.2013.03.017 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

