Impact of pathogen reduction methods on immunological properties of the COVID-19 convalescent plasma
- PMID: 33734455
- PMCID: PMC8250394
- DOI: 10.1111/vox.13056
Impact of pathogen reduction methods on immunological properties of the COVID-19 convalescent plasma
Abstract
Background and objectives: COVID-19 convalescent plasma is an experimental treatment against SARS-CoV-2. The aim of this study is to assess the impact of different pathogen reduction methods on the levels and virus neutralizing activity of the specific antibodies against SARS-CoV2 in convalescent plasma.
Materials and methods: A total of 140 plasma doses collected by plasmapheresis from COVID-19 convalescent donors were subjected to pathogen reduction by three methods: methylene blue (M)/visible light, riboflavin (R)/UVB and amotosalen (A)/UVA. To conduct a paired comparison, individual plasma doses were divided into 2 samples that were subjected to one of these methods. The titres of SARS-CoV2 neutralizing antibodies (NtAbs) and levels of specific immunoglobulins to RBD, S- and N-proteins of SARS-CoV-2 were measured before and after pathogen reduction.
Results: The methods reduced NtAbs titres differently: among units with the initial titre 80 or above, 81% of units remained unchanged and 19% decreased by one step after methylene blue; 60% were unchanged and 40% decreased by one step after amotosalen; after riboflavin 43% were unchanged and 50% (7%, respectively) had a one-step (two-step, respectively) decrease. Paired two-sample comparisons (M vs. A, M vs. R and A vs. R) revealed that the largest statistically significant decrease in quantity and activity of the specific antibodies resulted from the riboflavin treatment.
Conclusion: Pathogen reduction with methylene blue or with amotosalen provides the greater likelihood of preserving the immunological properties of the COVID-19 convalescent plasma compared to riboflavin.
Keywords: COVID-19 convalescent plasma; NtAbs; amotosalen; methylene blue; pathogen reduction; riboflavin.
© 2021 International Society of Blood Transfusion.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
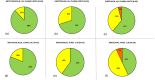
 ), one‐step titre decrease (
), one‐step titre decrease ( ) or two‐step titre decrease (
) or two‐step titre decrease ( ) after pathogen reduction with methylene blue (A, D), amotosalen (B, E) and riboflavin (C, F) among all plasma units (A, B, C) or only units with initial NtAbs titre 80 or above (D, E, F).
) after pathogen reduction with methylene blue (A, D), amotosalen (B, E) and riboflavin (C, F) among all plasma units (A, B, C) or only units with initial NtAbs titre 80 or above (D, E, F).References
-
- Lamontagne F, Agoritsas T, Macdonald H, et al. A living WHO guideline on drugs for covid‐19. BMJ 2020;370:m3379. - PubMed
-
- Luke TC, Kilbane EM, Jackson JL, et al. Meta‐analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 2006;145:599–609. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

