Periprostatic adipose tissue promotes prostate cancer resistance to docetaxel by paracrine IGF-1 upregulation of TUBB2B beta-tubulin isoform
- PMID: 33734457
- PMCID: PMC8251776
- DOI: 10.1002/pros.24117
Periprostatic adipose tissue promotes prostate cancer resistance to docetaxel by paracrine IGF-1 upregulation of TUBB2B beta-tubulin isoform
Abstract
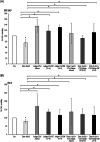
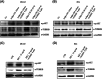
Growing evidence supports the pivotal role played by periprostatic adipose tissue (PPAT) in prostate cancer (PCa) microenvironment. We investigated whether PPAT can affect response to Docetaxel (DCTX) and the mechanisms associated. Conditioned medium was collected from the in vitro differentiated adipocytes isolated from PPAT which was isolated from PCa patients, during radical prostatectomy. Drug efficacy was studied by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide citotoxicity assay. Culture with CM of human PPAT (AdipoCM) promotes DCTX resistance in two different human prostate cancer cell lines (DU145 and PC3) and upregulated the expression of BCL-xL, BCL-2, and TUBB2B. AG1024, a well-known IGF-1 receptor inhibitor, counteracts the decreased response to DCTX observed in presence of AdipoCM and decreased TUBB2B expression, suggesting that a paracrine secretion of IGF-1 by PPAT affect DCTX response of PCa cell. Collectively, our study showed that factors secreted by PPAT elicits DCTX resistance through antiapoptotic proteins and TUBB2B upregulation in androgen independent PCa cell lines. These findings reveal the potential of novel therapeutic strategies targeting adipocyte-released factors and IGF-1 axis to overcome DCTX resistance in patients with PCa.
Keywords: adipocytes; docetaxel; drug resistance; periprostatic adipose tissue; prostate cancer.
© 2021 The Authors. The Prostate published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures




Similar articles
-
Tumoral periprostatic adipose tissue exovesicles-derived miR-20a-5p regulates prostate cancer cell proliferation and inflammation through the RORA gene.J Transl Med. 2024 Jul 15;22(1):661. doi: 10.1186/s12967-024-05458-3. J Transl Med. 2024. PMID: 39010137 Free PMC article.
-
Peri-Prostatic Adipocyte-Released TGFβ Enhances Prostate Cancer Cell Motility by Upregulation of Connective Tissue Growth Factor.Biomedicines. 2021 Nov 15;9(11):1692. doi: 10.3390/biomedicines9111692. Biomedicines. 2021. PMID: 34829922 Free PMC article.
-
Insulin-like growth factor-binding protein-2 promotes prostate cancer cell growth via IGF-dependent or -independent mechanisms and reduces the efficacy of docetaxel.Br J Cancer. 2011 May 10;104(10):1587-93. doi: 10.1038/bjc.2011.127. Epub 2011 Apr 12. Br J Cancer. 2011. PMID: 21487405 Free PMC article.
-
Periprostatic Adipose Tissue Microenvironment: Metabolic and Hormonal Pathways During Prostate Cancer Progression.Front Endocrinol (Lausanne). 2022 Apr 13;13:863027. doi: 10.3389/fendo.2022.863027. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35498409 Free PMC article. Review.
-
Interplay between Prostate Cancer and Adipose Microenvironment: A Complex and Flexible Scenario.Int J Mol Sci. 2022 Sep 15;23(18):10762. doi: 10.3390/ijms231810762. Int J Mol Sci. 2022. PMID: 36142673 Free PMC article. Review.
Cited by
-
Tumoral periprostatic adipose tissue exovesicles-derived miR-20a-5p regulates prostate cancer cell proliferation and inflammation through the RORA gene.J Transl Med. 2024 Jul 15;22(1):661. doi: 10.1186/s12967-024-05458-3. J Transl Med. 2024. PMID: 39010137 Free PMC article.
-
PPARG is a potential target of Tanshinone IIA in prostate cancer treatment: a combination study of molecular docking and dynamic simulation based on transcriptomic bioinformatics.Eur J Med Res. 2023 Nov 6;28(1):487. doi: 10.1186/s40001-023-01477-w. Eur J Med Res. 2023. PMID: 37932808 Free PMC article.
-
Investigating periprostatic adipose tissue as a driving force of prostate cancer progression: a new source of information for the advancement of targeted therapy in metastatic prostate cancer.J Basic Clin Physiol Pharmacol. 2023 Mar 27;34(3):245-247. doi: 10.1515/jbcpp-2023-0059. eCollection 2023 May 1. J Basic Clin Physiol Pharmacol. 2023. PMID: 36972321 No abstract available.
-
Adipose Tissues Have Been Overlooked as Players in Prostate Cancer Progression.Int J Mol Sci. 2024 Nov 12;25(22):12137. doi: 10.3390/ijms252212137. Int J Mol Sci. 2024. PMID: 39596205 Free PMC article. Review.
-
Overexpressed KCNK1 regulates potassium channels affecting molecular mechanisms and biological pathways in bladder cancer.Eur J Med Res. 2024 Apr 30;29(1):257. doi: 10.1186/s40001-024-01844-1. Eur J Med Res. 2024. PMID: 38689322 Free PMC article.
References
-
- McMillin DW, Negri JM, Mitsiades CS. The role of tumour‐stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Discov. 2013;12(3):217‐228. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

