A hypomyelinating leukodystrophy in German Shepherd dogs
- PMID: 33734486
- PMCID: PMC8163122
- DOI: 10.1111/jvim.16085
A hypomyelinating leukodystrophy in German Shepherd dogs
Abstract
Background: Shaking puppy syndrome is commonly attributed to abnormal myelination of the central nervous system.
Hypothesis/objectives: To report the long-term clinical course and the imaging characteristics of hypomyelinating leukodystrophy in German Shepherd dogs.
Animals and methods: Three related litters with 11 affected dogs.
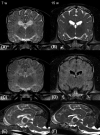
Results: The 11 affected dogs experienced coarse, side-to-side tremors of the head and trunk, which interfered with normal goal-oriented movements and disappeared at rest. Signs were noticed shortly after birth. Nine dogs were euthanized, 3 dogs underwent pathological examination, and 2 littermates were raised by their breeder. Tremors improved gradually until 6 to 7 months of age. Adult dogs walked with severe residual pelvic limb ataxia. One dog developed epilepsy with tonic-clonic seizures at 15 months of age. Conventional magnetic resonance imaging (MRI) disclosed homogenous hyperintense signal of the entire subcortical white matter in 3 affected 7-week-old dogs and a hypointense signal in a presumably unaffected littermate. Subcortical white matter appeared isointense to gray matter at 15 and 27 weeks of age on repeated MRI. Abnormal white matter signal with failure to display normal gray-white matter contrast persisted into adulthood. Cerebellar arbor vitae was not visible at any time point. Clinical signs, MRI findings, and pathological examinations were indicative of a hypomyelinating leukodystrophy. All parents of the affected litters shared a common ancestor and relatedness of the puppies suggested an autosomal recessive mode of inheritance.
Conclusion: We describe a novel hypomyelinating leukodystrophy in German Shepherd dogs with a suspected inherited origin.
Keywords: animal model; brain maturation; development; dysmyelination; genetic; hypomyelination; inherited; leukoencephalopathy; seizures; tremor; white matter.
© 2021 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC. on behalf of the American College of Veterinary Internal Medicine.
Conflict of interest statement
Authors declare no conflict of interest.
Figures






References
-
- Duncan ID. Abnormalities of myelination of the central nervous system associated with congenital tremor. J Vet Intern Med. 1987;1:10‐23. - PubMed
-
- Cummings JF, Summers BA, de Lahunta A, Lawson C. Tremors in Samoyed pups with oligodendrocyte deficiencies and hypomyelination. Acta Neuropathol. 1986;71:267‐277. - PubMed
-
- Palmer AC, Blakemore WF, Wallace ME, Wilkes MK, Herrtage ME, Matic SE. Recognition of 'trembler', a hypomyelinating condition in the Bernese mountain dog. Vet Rec. 1987;120:609‐612. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
