Analysing the attributes of Comprehensive Cancer Centres and Cancer Centres across Europe to identify key hallmarks
- PMID: 33734563
- PMCID: PMC8096787
- DOI: 10.1002/1878-0261.12950
Analysing the attributes of Comprehensive Cancer Centres and Cancer Centres across Europe to identify key hallmarks
Abstract
There is a persistent variation in cancer outcomes among and within European countries suggesting (among other causes) inequalities in access to or delivery of high-quality cancer care. European policy (EU Cancer Mission and Europe's Beating Cancer Plan) is currently moving towards a mission-oriented approach addressing these inequalities. In this study, we used the quantitative and qualitative data of the Organisation of European Cancer Institutes' Accreditation and Designation Programme, relating to 40 large European cancer centres, to describe their current compliance with quality standards, to identify the hallmarks common to all centres and to show the distinctive features of Comprehensive Cancer Centres. All Comprehensive Cancer Centres and Cancer Centres accredited by the Organisation of European Cancer Institutes show good compliance with quality standards related to care, multidisciplinarity and patient centredness. However, Comprehensive Cancer Centres on average showed significantly better scores on indicators related to the volume, quality and integration of translational research, such as high-impact publications, clinical trial activity (especially in phase I and phase IIa trials) and filing more patents as early indicators of innovation. However, irrespective of their size, centres show significant variability regarding effective governance when functioning as entities within larger hospitals.
Keywords: accreditation; clinical trials; comprehensive cancer center; multidisciplinarity; quality standard; translational research.
© 2021 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
SK, SO and AW received funding from the Organisation of European Cancer Institutes for this work. WvH receives a nonrestricted grant from Novartis, a nonrestricted grant from Agendia BV and a nonrestricted grant from Intuitive Surgical. PN and JL acknowledge financial support from the Hungarian Thematic Excellence Programme (TKP2020‐NKA‐26). All remaining authors have declared no conflicts of interest.
Figures



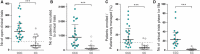
References
-
- Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O & Bray F (2018) Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 103, 356–387. - PubMed
-
- European Commission. Conquering cancer: Mission Possible. Interim Report of the Mission Board for Cancer. Publications office of the European Union, Luxembourg, 2020. https://op.europa.eu/en/web/eu‐law‐and‐publications/publication‐detail/‐... (accessed 26 June 2020).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

