2,4,5-Trisubstituted Pyrimidines as Potent HIV-1 NNRTIs: Rational Design, Synthesis, Activity Evaluation, and Crystallographic Studies
- PMID: 33734714
- PMCID: PMC8594587
- DOI: 10.1021/acs.jmedchem.1c00268
2,4,5-Trisubstituted Pyrimidines as Potent HIV-1 NNRTIs: Rational Design, Synthesis, Activity Evaluation, and Crystallographic Studies
Abstract
There is an urgent unmet medical need for novel human immunodeficiency virus type 1 (HIV-1) inhibitors that are effective against a variety of NNRTI-resistance mutations. We report our research efforts aimed at discovering a novel chemotype of anti-HIV-1 agents with improved potency against a variety of NNRTI-resistance mutations in this paper. Structural modifications of the lead K-5a2 led to the identification of a potent inhibitor 16c. 16c yielded highly potent anti-HIV-1 activities and improved resistance profiles compared with the approved drug etravirine. The co-crystal structure revealed the key role of the water networks surrounding the NNIBP for binding and for resilience against resistance mutations, while suggesting further extension of 16c toward the NNRTI-adjacent site as a lead development strategy. Furthermore, 16c demonstrated favorable pharmacokinetic and safety properties, suggesting the potential of 16c as a promising anti-HIV-1 drug candidate.
Figures
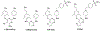
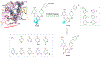
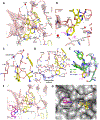
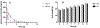

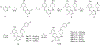
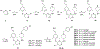
References
-
- Bec G; Meyer B; Gerard M-A; Steger J; Fauster K; Wolff P; Burnouf D; Micura R; Dumas P; Ennifar E Thermodynamics of HIV-1 reverse transcriptase in action elucidates the mechanism of action of non-nucleoside inhibitors. J. Am. Chem. Soc 2013, 135, 9743–9752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical

