Advances in the Design of (Nano)Formulations for Delivery of Antisense Oligonucleotides and Small Interfering RNA: Focus on the Central Nervous System
- PMID: 33734715
- PMCID: PMC8824433
- DOI: 10.1021/acs.molpharmaceut.0c01238
Advances in the Design of (Nano)Formulations for Delivery of Antisense Oligonucleotides and Small Interfering RNA: Focus on the Central Nervous System
Abstract
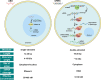
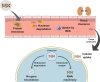
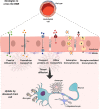
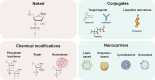
RNA-based therapeutics have emerged as one of the most powerful therapeutic options used for the modulation of gene/protein expression and gene editing with the potential to treat neurodegenerative diseases. However, the delivery of nucleic acids to the central nervous system (CNS), in particular by the systemic route, remains a major hurdle. This review will focus on the strategies for systemic delivery of therapeutic nucleic acids designed to overcome these barriers. Pathways and mechanisms of transport across the blood-brain barrier which could be exploited for delivery are described, focusing in particular on smaller nucleic acids including antisense oligonucleotides (ASOs) and small interfering RNA (siRNA). Approaches used to enhance delivery including chemical modifications, nanocarrier systems, and target selection (cell-specific delivery) are critically analyzed. Learnings achieved from a comparison of the successes and failures reported for CNS delivery of ASOs versus siRNA will help identify opportunities for a wider range of nucleic acids and accelerate the clinical translation of these innovative therapies.
Keywords: antisense oligonucleotide; blood−brain barrier; neurological diseases; small interfering RNA; systemic delivery.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Li M.; Snider B. J.. Gene Therapy Methods and Their Applications in Neurological Disorders. In Gene Therapy in Neurological Disorders; Li M., Snider B. J., Eds.; Elsevier Inc., 2018; pp 3–39, 10.1016/B978-0-12-809813-4.00001-6. - DOI
-
- Han Z.Gene Therapy Using Genomic DNA: Advances and Challenges. In Gene Therapy in Neurological Disorders; Li M., Snider B. J., Eds.; Elsevier Inc., 2018; pp 63–80, 10.1016/B978-0-12-809813-4.00003-X. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

