COVID-19 Response Efforts of Washington State Public Health Laboratory: Lessons Learned
- PMID: 33734847
- PMCID: PMC8034017
- DOI: 10.2105/AJPH.2021.306212
COVID-19 Response Efforts of Washington State Public Health Laboratory: Lessons Learned
Abstract
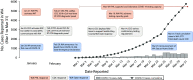
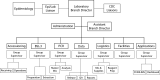
Laboratory diagnostics play an essential role in pandemic preparedness. In January 2020, the first US case of COVID-19 was confirmed in Washington State. At the same time, the Washington State Public Health Laboratory (WA PHL) was in the process of building upon and initiating innovative preparedness activities to strengthen laboratory testing capabilities, operations, and logistics. The response efforts of WA PHL, in conjunction with the Centers for Disease Control and Prevention, to the COVID-19 outbreak in Washington are described herein-from the initial detection of severe acute respiratory syndrome coronavirus 2 through the subsequent 2 months.Factors that contributed to an effective laboratory response are described, including preparing early to establish testing capacity, instituting dynamic workforce solutions, advancing information management systems, refining laboratory operations, and leveraging laboratory partnerships. We also report on the challenges faced, successful steps taken, and lessons learned by WA PHL to respond to COVID-19.The actions taken by WA PHL to mount an effective public health response may be useful for US laboratories as they continue to respond to the COVID-19 pandemic and may help inform current and future laboratory pandemic preparedness activities.
Figures

References
-
- Washington State Department of Health. Novel Coronavirus Outbreak (COVID-19) 2019. Available at: https://www.doh.wa.gov/Emergencies/NovelCoronavirusOutbreak2020COVID19/D.... Accessed July 24, 2020.
-
- Centers for Disease Control and Prevention. 2019-nCoV Real Time RT-PCR Diagnostic Panel instructions for use. 2020. Available at: https://www.fda.gov/media/134922/download. Accessed July 24, 2020.
-
- Centers for Disease Control and Prevention. Revision to test instructions. CDC 2019 Novel Coronavirus (nCoV) Real-Time RT-PCR Diagnostic Panel (EUA200001) Available at: https://www.aphl.org/Materials/Signed_CDC_Letter_to_PHLs-N3_Removal_Inst.... Accessed July 24, 2020.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

