Low-Dose Tamoxifen for Mammographic Density Reduction: A Randomized Controlled Trial
- PMID: 33734864
- PMCID: PMC8189632
- DOI: 10.1200/JCO.20.02598
Low-Dose Tamoxifen for Mammographic Density Reduction: A Randomized Controlled Trial
Abstract
Purpose: Tamoxifen prevents breast cancer in high-risk women and reduces mortality in the adjuvant setting. Mammographic density change is a proxy for tamoxifen therapy response. We tested whether lower doses of tamoxifen were noninferior to reduce mammographic density and associated with fewer symptoms.
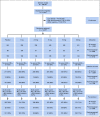
Patients and methods: Women, 40-74 years of age, participating in the Swedish mammography screening program were invited to the 6-month double-blind six-arm randomized placebo-controlled noninferiority dose-determination KARISMA phase II trial stratified by menopausal status (EudraCT 2016-000882-22). In all, 1,439 women were accrued with 1,230 participants accessible for intention-to-treat analysis. The primary outcome was proportion of women treated with placebo, 1, 2.5, 5, and 10 mg whose mammographic density decreased at least as much as the median reduction in the 20 mg arm. The noninferior margin was 17%. Secondary outcome was reduction of symptoms. Post hoc analyses were performed by menopausal status. Per-protocol population and full population were analyzed in sensitivity analysis.
Results: The 1,439 participants, 566 and 873 pre- and postmenopausal women, respectively, were recruited between October 1, 2016, and September 30, 2019. The participants had noninferior mammographic density reduction following 2.5, 5, and 10 mg tamoxifen compared with the median 10.1% decrease observed in the 20 mg group, a reduction confined to premenopausal women. Severe vasomotor symptoms (hot flashes, cold sweats, and night sweats) were reduced by approximately 50% in the 2.5, 5, and 10 mg groups compared with the 20 mg group.
Conclusion: Premenopausal women showed noninferior magnitude of breast density decrease at 2.5 mg of tamoxifen, but fewer side effects compared with the standard dose of 20 mg. Future studies should test whether 2.5 mg of tamoxifen reduces the risk of primary breast cancer.
Conflict of interest statement
Figures

Comment in
-
Regarding the Appropriate Target and Duration of Chemoprevention in Breast Cancer.J Clin Oncol. 2021 Sep 10;39(26):2965-2966. doi: 10.1200/JCO.21.01060. Epub 2021 Jun 14. J Clin Oncol. 2021. PMID: 34125580 No abstract available.
-
Reply to T. Suemasu et al.J Clin Oncol. 2021 Sep 10;39(26):2966-2968. doi: 10.1200/JCO.21.01166. Epub 2021 Jun 14. J Clin Oncol. 2021. PMID: 34125585 No abstract available.
References
-
- American Cancer Society : Breast Cancer Facts & Figures 2019-2020. Atlanta, GA, American Cancer Society, 2019
-
- Fisher B Costantino JP Wickerham DL, et al. : Tamoxifen for the prevention of breast cancer: Current status of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst 97:1652-1662, 2005 - PubMed
-
- Powles TJ Ashley S Tidy A, et al. : Twenty year follow-up of the Royal Marsden randomized, double blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst 99:283-290, 2007 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

