Prospective Validation of CD-62L (L-Selectin) as Marker of Durable Response to Infliximab Treatment in Patients With Inflammatory Bowel Disease: A 5-Year Clinical Follow-up
- PMID: 33735154
- PMCID: PMC7886452
- DOI: 10.14309/ctg.0000000000000298
Prospective Validation of CD-62L (L-Selectin) as Marker of Durable Response to Infliximab Treatment in Patients With Inflammatory Bowel Disease: A 5-Year Clinical Follow-up
Abstract
Introduction: The development of biomarkers to guide management of anti-tumor necrosis factor (TNF) agents in patients with inflammatory bowel disease (IBD) is an unmet need. We developed an in vitro blood assay to predict patient long-term outcome with the anti-TNFα agent infliximab (IFX).
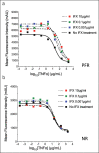
Methods: Patients with IBD were classified according to the shedding of an L-selectin (CD62L) from the surface of their granulocytes in whole blood. CD62L shedding was quantified by flow cytometry before and after drug administration. A clinical data collection from June 2012 to August 2017 with blinded IFX management was aimed at validating the long-term predictive value of this test.
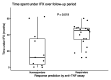
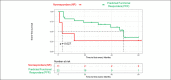
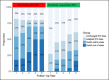
Results: Among 33 patients with IBD (17 Crohn's disease and 5 ulcerative colitis), 22 were predicted functional responders (PFR) and 11 were predicted as nonresponders (NR) according to the in vitro test. Five years after study initiation, 72% of PFR were still treated with IFX (vs 27% in the NR group; P < 0.05), with a median time spent under IFX of 45 vs 12 months (P = 0.019), respectively. Thirty-five medicosurgical events occurred with a median time to first event of 3 vs 30 months (P = 0.023), respectively. Our assay was the best independent predictor of staying long term on IFX (P = 0.056).
Discussion: An assay-based in vitro test for functional blockade of TNFα (CD62L shedding) provides an excellent long-term (at 3-5 years) independent predictor of durable use of IFX in patients with IBD. Testing patients could personalize decision making to significantly reduce costs and risk of adverse events and complications.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures
Similar articles
-
Association Between Infliximab Drug and Antibody Levels and Therapy Outcome in Pediatric Inflammatory Bowel Diseases.J Pediatr Gastroenterol Nutr. 2018 Oct;67(4):507-512. doi: 10.1097/MPG.0000000000002051. J Pediatr Gastroenterol Nutr. 2018. PMID: 29901557
-
Infliximab Optimization Based on Therapeutic Drug Monitoring in Pediatric Inflammatory Bowel Disease.J Pediatr Gastroenterol Nutr. 2017 Apr;64(4):580-585. doi: 10.1097/MPG.0000000000001302. J Pediatr Gastroenterol Nutr. 2017. PMID: 28079601
-
Clinical Use of Measuring Trough Levels and Antibodies against Infliximab in Patients with Pediatric Inflammatory Bowel Disease.Gut Liver. 2017 Jan 15;11(1):55-61. doi: 10.5009/gnl16041. Gut Liver. 2017. PMID: 27609485 Free PMC article.
-
Management of inflammatory bowel disease with infliximab and other anti-tumor necrosis factor alpha therapies.BioDrugs. 2010 Dec 14;24 Suppl 1:3-14. doi: 10.2165/11586290-000000000-00000. BioDrugs. 2010. PMID: 21175228 Review.
-
Review article: why, when and how to de-escalate therapy in inflammatory bowel diseases.Aliment Pharmacol Ther. 2014 Aug;40(4):338-53. doi: 10.1111/apt.12838. Epub 2014 Jun 23. Aliment Pharmacol Ther. 2014. PMID: 24957164 Review.
Cited by
-
Novel biomarkers related to oxidative stress and immunity in chronic kidney disease.Heliyon. 2024 Mar 12;10(6):e27754. doi: 10.1016/j.heliyon.2024.e27754. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38515668 Free PMC article.
-
A Series of Genes for Predicting Responses to Anti-Tumor Necrosis Factor α Therapy in Crohn's Disease.Front Pharmacol. 2022 Apr 20;13:870796. doi: 10.3389/fphar.2022.870796. eCollection 2022. Front Pharmacol. 2022. PMID: 35517818 Free PMC article.
-
Tackling Inflammatory Bowel Diseases: Targeting Proinflammatory Cytokines and Lymphocyte Homing.Pharmaceuticals (Basel). 2022 Aug 30;15(9):1080. doi: 10.3390/ph15091080. Pharmaceuticals (Basel). 2022. PMID: 36145301 Free PMC article. Review.
References
-
- Kaplan GG. The global burden of IBD: From 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015;12:720. - PubMed
-
- Torres J, Mehandru S, Colombel JF, et al. Crohn's disease. Lancet 2017;389(10080):1741–55. - PubMed
-
- Hoivik ML, Moum B, Solberg IC, et al. Work disability in inflammatory bowel disease patients 10 years after disease onset: Results from the IBSEN study. Gut 2013;62(3):368–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

