Effectiveness of the Pfizer-BioNTech COVID-19 Vaccine Among Residents of Two Skilled Nursing Facilities Experiencing COVID-19 Outbreaks - Connecticut, December 2020-February 2021
- PMID: 33735160
- PMCID: PMC7976620
- DOI: 10.15585/mmwr.mm7011e3
Effectiveness of the Pfizer-BioNTech COVID-19 Vaccine Among Residents of Two Skilled Nursing Facilities Experiencing COVID-19 Outbreaks - Connecticut, December 2020-February 2021
Abstract
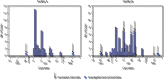
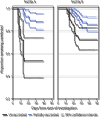
Residents of long-term care facilities (LTCFs), particularly those in skilled nursing facilities (SNFs), have experienced disproportionately high levels of COVID-19-associated morbidity and mortality and were prioritized for early COVID-19 vaccination (1,2). However, this group was not included in COVID-19 vaccine clinical trials, and limited postauthorization vaccine effectiveness (VE) data are available for this critical population (3). It is not known how well COVID-19 vaccines protect SNF residents, who typically are more medically frail, are older, and have more underlying medical conditions than the general population (1). In addition, immunogenicity of the Pfizer-BioNTech vaccine was found to be lower in adults aged 65-85 years than in younger adults (4). Through the CDC Pharmacy Partnership for Long-Term Care Program, SNF residents and staff members in Connecticut began receiving the Pfizer-BioNTech COVID-19 vaccine on December 18, 2020 (5). Administration of the vaccine was conducted during several on-site pharmacy clinics. In late January 2021, the Connecticut Department of Public Health (CT DPH) identified two SNFs experiencing COVID-19 outbreaks among residents and staff members that occurred after each facility's first vaccination clinic. CT DPH, in partnership with CDC, performed electronic chart review in these facilities to obtain information on resident vaccination status and infection with SARS-CoV-2, the virus that causes COVID-19. Partial vaccination, defined as the period from >14 days after the first dose through 7 days after the second dose, had an estimated effectiveness of 63% (95% confidence interval [CI] = 33%-79%) against SARS-CoV-2 infection (regardless of symptoms) among residents within these SNFs. This is similar to estimated effectiveness for a single dose of the Pfizer-BioNTech COVID-19 vaccine in adults across a range of age groups in noncongregate settings (6) and suggests that to optimize vaccine impact among this population, high coverage with the complete 2-dose series should be recommended for SNF residents and staff members.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- Gharpure R, Guo A, Bishnoi CK, et al. Early COVID-19 first-dose vaccination coverage among residents and staff members of skilled nursing facilities participating in the Pharmacy Partnership for Long-Term Care Program—United States, December 2020–January 2021. MMWR Morb Mortal Wkly Rep 2021;70:178–82. 10.15585/mmwr.mm7005e2 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

