COVID-19 Vaccine Second-Dose Completion and Interval Between First and Second Doses Among Vaccinated Persons - United States, December 14, 2020-February 14, 2021
- PMID: 33735162
- PMCID: PMC7976616
- DOI: 10.15585/mmwr.mm7011e2
COVID-19 Vaccine Second-Dose Completion and Interval Between First and Second Doses Among Vaccinated Persons - United States, December 14, 2020-February 14, 2021
Abstract
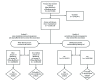
In December 2020, two COVID-19 vaccines (Pfizer-BioNTech and Moderna) received Emergency Use Authorization from the Food and Drug Administration.*,† Both vaccines require 2 doses for a completed series. The recommended interval between doses is 21 days for Pfizer-BioNTech and 28 days for Moderna; however, up to 42 days between doses is permissible when a delay is unavoidable.§ Two analyses of COVID-19 vaccine administration data were conducted among persons who initiated the vaccination series during December 14, 2020-February 14, 2021, and whose doses were reported to CDC through February 20, 2021. The first analysis was conducted to determine whether persons who received a first dose and had sufficient time to receive the second dose (i.e., as of February 14, 2021, >25 days from receipt of Pfizer-BioNTech vaccine or >32 days from receipt of Moderna vaccine had elapsed) had received the second dose. A second analysis was conducted among persons who received a second COVID-19 dose by February 14, 2021, to determine whether the dose was received during the recommended dosing interval, which in this study was defined as 17-25 days (Pfizer-BioNTech) and 24-32 days (Moderna) after the first dose. Analyses were stratified by jurisdiction and by demographic characteristics. In the first analysis, among 12,496,258 persons who received the first vaccine dose and for whom sufficient time had elapsed to receive the second dose, 88.0% had completed the series, 8.6% had not received the second dose but remained within the allowable interval (≤42 days since the first dose), and 3.4% had missed the second dose (outside the allowable interval, >42 days since the first dose). The percentage of persons who missed the second dose varied by jurisdiction (range = 0.0%-9.1%) and among demographic groups was highest among non-Hispanic American Indian/Alaska Native (AI/AN) persons (5.1%) and persons aged 16-44 years (4.0%). In the second analysis, among 14,205,768 persons who received a second dose, 95.6% received the dose within the recommended interval, although percentages varied by jurisdiction (range = 79.0%-99.9%). Public health officials should identify and address possible barriers to completing the COVID-19 vaccination series to ensure equitable coverage across communities and maximum health benefits for recipients. Strategies to ensure series completion could include scheduling second-dose appointments at the first-dose administration and sending reminders for second-dose visits.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

