Regulation of sperm motility in Eastern oyster (Crassostrea virginica) spawning naturally in seawater with low salinity
- PMID: 33735238
- PMCID: PMC7971463
- DOI: 10.1371/journal.pone.0243569
Regulation of sperm motility in Eastern oyster (Crassostrea virginica) spawning naturally in seawater with low salinity
Abstract
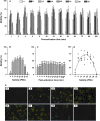
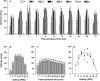
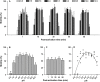
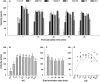
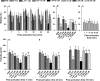
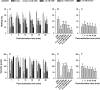
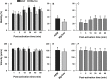
Oyster aquaculture is expanding worldwide, where many farms rely on seed produced by artificial spawning. As sperm motility and velocity are key determinants for fertilization success, understanding the regulation of sperm motility and identifying optimal environmental conditions can increase fertility and seed production. In the present study, we investigated the physiological mechanisms regulating sperm motility in Eastern oyster, Crassostrea virginica. Sperm motility was activated in ambient seawater with salinity 4-32 PSU with highest motility and velocity observed at 12-24 PSU. In artificial seawater (ASW) with salinity of 20 PSU, sperm motility was activated at pH 6.5-10.5 with the highest motility and velocity recorded at pH 7.5-10.0. Sperm motility was inhibited or totally suppressed in Na+, K+, Ca2+, and Mg2+-free ASW at 20 PSU. Applications of K+ (500 μM glybenclamide and 10-50 mM 4-aminopyridine), Ca2+ (1-50 μM mibefradil and 10-200 μM verapamil), or Na+ (0.2-2.0 mM amiloride) channel blockers into ASW at 20 PSU inhibited or suppressed sperm motility and velocity. Chelating extracellular Ca2+ ions by 3.0 and 3.5 mM EGTA resulted in a significant reduction and full suppression of sperm motility by 4 to 6 min post-activation. These results suggest that extracellular K+, Ca2+, and Na+ ions are involved in regulation of ionic-dependent sperm motility in Eastern oyster. A comparison with other bivalve species typically spawning at higher salinities or in full-strength seawater shows that ionic regulation of sperm motility is physiologically conserved in bivalves. Elucidating sperm regulation in C. virginica has implications to develop artificial reproduction, sperm short-term storage, or cryopreservation protocols, and to better predict how changes in the ocean will impact oyster spawning dynamics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Roles of extracellular ions and pH in 5-HT-induced sperm motility in marine bivalve.Reproduction. 2014 Feb 3;147(3):331-45. doi: 10.1530/REP-13-0418. Print 2014 Mar. Reproduction. 2014. PMID: 24398874
-
Serotonin-Induced Sperm Hyper-Motility In Pacific Oyster (Crassostrea Gigas) Associates With K+ Efflux and Membrane Hyperpolarization.J Exp Zool A Ecol Integr Physiol. 2025 Jul;343(6):677-692. doi: 10.1002/jez.2918. Epub 2025 Apr 9. J Exp Zool A Ecol Integr Physiol. 2025. PMID: 40200823
-
pH controls spermatozoa motility in the Pacific oyster (Crassostrea gigas).Biol Open. 2018 Mar 19;7(3):bio031427. doi: 10.1242/bio.031427. Biol Open. 2018. PMID: 29483075 Free PMC article.
-
Sperm motility in fishes. (II) Effects of ions and osmolality: a review.Cell Biol Int. 2006 Jan;30(1):1-14. doi: 10.1016/j.cellbi.2005.06.004. Epub 2005 Nov 8. Cell Biol Int. 2006. PMID: 16278089 Review.
-
Sperm motility in fishes: (III) diversity of regulatory signals from membrane to the axoneme.Theriogenology. 2019 Sep 15;136:143-165. doi: 10.1016/j.theriogenology.2019.06.038. Epub 2019 Jun 25. Theriogenology. 2019. PMID: 31265944 Review.
Cited by
-
Hdh-Tektin-4 Regulates Motility of Fresh and Cryopreserved Sperm in Pacific Abalone, Haliotis discus hannai.Front Cell Dev Biol. 2022 Apr 25;10:870743. doi: 10.3389/fcell.2022.870743. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35547812 Free PMC article.
-
Unravelling the ecotoxicological impacts of gadolinium (Gd) on Mytilus galloprovincialis embryos and sperm in seawater: A preliminary study.Heliyon. 2024 May 17;10(10):e31087. doi: 10.1016/j.heliyon.2024.e31087. eCollection 2024 May 30. Heliyon. 2024. PMID: 38826730 Free PMC article.
-
Polystyrene Nanoplastics in Aquatic Microenvironments Affect Sperm Metabolism and Fertilization of Mytilus galloprovincialis (Lamark, 1819).Toxics. 2023 Nov 11;11(11):924. doi: 10.3390/toxics11110924. Toxics. 2023. PMID: 37999576 Free PMC article.
-
Effects of Ammonia Concentration on Sperm Vitality, Motility Rates, and Morphology in Three Marine Bivalve Species: A Comparative Study of the Noble Scallop Mimachlamys nobilis, Chinese Pearl Oyster Pinctada fucata martensii, and Small Rock Oyster Saccostrea mordax.Biology (Basel). 2024 Aug 3;13(8):589. doi: 10.3390/biology13080589. Biology (Basel). 2024. PMID: 39194527 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

