Sulforaphane (SFA) protects neuronal cells from oxygen & glucose deprivation (OGD)
- PMID: 33735260
- PMCID: PMC7971874
- DOI: 10.1371/journal.pone.0248777
Sulforaphane (SFA) protects neuronal cells from oxygen & glucose deprivation (OGD)
Abstract
Background: Perinatal brain injury results in neurodevelopmental disabilities (neuroDDs) that include cerebral palsy, autism, attention deficit disorder, epilepsy, learning disabilities and others. Commonly, injury occurs when placental circulation, that is responsible for transporting nutrients and oxygen to the fetus, is compromised. Placental insufficiency (PI) is a reduced supply of blood and oxygen to the fetus and results in a hypoxic-ischemic (HI) environment. A significant HI state in-utero leads to perinatal compromise, characterized by fetal growth restriction and brain injury. Given that over 80% of perinatal brain injuries that result in neuroDDs occur during gestation, prior to birth, preventive approaches are needed to reduce or eliminate the potential for injury and subsequent neuroDDs. Sulforaphane (SFA) derived from cruciferous vegetables such as broccoli sprouts (BrSps) is a phase-II enzyme inducer that acts via cytoplasmic Nrf2 to enhance the production of anti-oxidants in the brain through the glutathione pathway. We have previously shown a profound in vivo neuro-protective effect of BrSps/SFA as a dietary supplement in pregnant rat models of both PI and fetal inflammation. Strong evidence also points to a role for SFA as treatment for various cancers. Paradoxically, then SFA has the ability to enhance cell survival, and with conditions of cancer, enhance cell death. Given our findings of the benefit of SFA/Broccoli Sprouts as a dietary supplement during pregnancy, with improvement to the fetus, it is important to determine the beneficial and toxic dosing range of SFA. We therefore explored, in vitro, the dosing range of SFA for neuronal and glial protection and toxicity in normal and oxygen/glucose deprived (OGD) cell cultures.
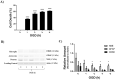
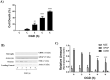
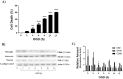
Methods: OGD simulates, in vitro, the condition experienced by the fetal brain due to PI. We developed a cell culture model of primary cortical neuronal, astrocyte and combined brain cell co-cultures from newborn rodent brains. The cultures were exposed to an OGD environment for various durations of time to determine the LD50 (duration of OGD required for 50% cell death). Using the LD50 as the time point, we evaluated the efficacy of varying doses of SFA for neuroprotective and neurotoxicity effects. Control cultures were exposed to normal media without OGD, and cytotoxicity of varying doses of SFA was also evaluated. Immunofluorescence (IF) and Western blot analysis of cell specific markers were used for culture characterization, and quantification of LD50. Efficacy and toxicity effect of SFA was assessed by IF/high content microscopy and by AlamarBlue viability assay, respectively.
Results: We determined the LD50 to be 2 hours for neurons, 8 hours for astrocytes, and 10 hours for co-cultures. The protective effect of SFA was noticeable at 2.5 μM and 5 μM for neurons, although it was not significant. There was a significant protective effect of SFA at 2.5 μM (p<0.05) for astrocytes and co-cultures. Significant toxicity ranges were also confirmed in OGD cultures as ≥ 100 μM (p<0.05) for astrocytes, ≥ 50 μM (p<0.01) for co-cultures, but not toxic in neurons; and toxic in control cultures as ≥ 100 μM (p<0.01) for neurons, and ≥ 50 μM (p<0.01) for astrocytes and co-cultures. One Way ANOVA and Dunnett's Multiple Comparison Test were used for statistical analysis.
Conclusions: Our results indicate that cell death shows a trend to reduction in neuronal and astrocyte cultures, and is significantly reduced in co-cultures treated with low doses of SFA exposed to OGD. Doses of SFA that were 10 times higher were toxic, not only under conditions of OGD, but in normal control cultures as well. The findings suggest that: 1. SFA shows promise as a preventative agent for fetal ischemic brain injury, and 2. Because the fetus is a rapidly growing organism with profound cell multiplication, dosing parameters must be established to insure safety within efficacious ranges. This study will influence the development of innovative therapies for the prevention of childhood neuroDD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

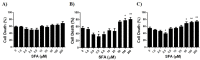

Similar articles
-
Monocarboxylate transporter-dependent mechanism confers resistance to oxygen- and glucose-deprivation injury in astrocyte-neuron co-cultures.Neurosci Lett. 2015 May 6;594:99-104. doi: 10.1016/j.neulet.2015.03.062. Epub 2015 Mar 28. Neurosci Lett. 2015. PMID: 25827488
-
The role of HIF in cobalt-induced ischemic tolerance.Neuroscience. 2013 Nov 12;252:420-30. doi: 10.1016/j.neuroscience.2013.07.060. Epub 2013 Aug 3. Neuroscience. 2013. PMID: 23916558
-
Features of the cytoprotective effect of selenium nanoparticles on primary cortical neurons and astrocytes during oxygen-glucose deprivation and reoxygenation.Sci Rep. 2022 Feb 2;12(1):1710. doi: 10.1038/s41598-022-05674-1. Sci Rep. 2022. PMID: 35110605 Free PMC article.
-
Sulforaphane and Brain Health: From Pathways of Action to Effects on Specific Disorders.Nutrients. 2025 Apr 15;17(8):1353. doi: 10.3390/nu17081353. Nutrients. 2025. PMID: 40284217 Free PMC article. Review.
-
The biological basis of injury and neuroprotection in the fetal and neonatal brain.Int J Dev Neurosci. 2011 Oct;29(6):551-63. doi: 10.1016/j.ijdevneu.2011.04.004. Epub 2011 Apr 15. Int J Dev Neurosci. 2011. PMID: 21527338 Free PMC article. Review.
Cited by
-
Sulforaphane protects developing neural networks from VPA-induced synaptic alterations.Mol Psychiatry. 2025 Sep;30(9):3868-3884. doi: 10.1038/s41380-025-02967-5. Epub 2025 Apr 2. Mol Psychiatry. 2025. PMID: 40175519 Free PMC article.
-
The role of isothiocyanate-rich plants and supplements in neuropsychiatric disorders: a review and update.Front Nutr. 2024 Sep 30;11:1448130. doi: 10.3389/fnut.2024.1448130. eCollection 2024. Front Nutr. 2024. PMID: 39421616 Free PMC article. Review.
-
Rescuing mitochondria in traumatic brain injury and intracerebral hemorrhages - A potential therapeutic approach.Neurochem Int. 2021 Nov;150:105192. doi: 10.1016/j.neuint.2021.105192. Epub 2021 Sep 22. Neurochem Int. 2021. PMID: 34560175 Free PMC article. Review.
-
miR-432-5p Inhibits the Ferroptosis in Cardiomyocytes Induced by Hypoxia/Reoxygenation via Activating Nrf2/SLC7A11 Axis by Degrading Keap1.Anal Cell Pathol (Amst). 2023 Oct 3;2023:1293200. doi: 10.1155/2023/1293200. eCollection 2023. Anal Cell Pathol (Amst). 2023. PMID: 37822721 Free PMC article.
-
Sulforaphane's Multifaceted Potential: From Neuroprotection to Anticancer Action.Molecules. 2023 Oct 1;28(19):6902. doi: 10.3390/molecules28196902. Molecules. 2023. PMID: 37836745 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

