Anti-CD19 CAR T cells potently redirected to kill solid tumor cells
- PMID: 33735268
- PMCID: PMC7971483
- DOI: 10.1371/journal.pone.0247701
Anti-CD19 CAR T cells potently redirected to kill solid tumor cells
Abstract
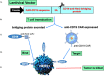
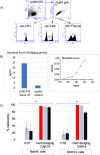
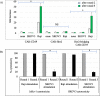
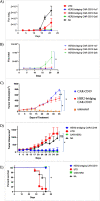
Successful CAR T cell therapy for the treatment of solid tumors requires exemplary CAR T cell expansion, persistence and fitness, and the ability to target tumor antigens safely. Here we address this constellation of critical attributes for successful cellular therapy by using integrated technologies that simplify development and derisk clinical translation. We have developed a CAR-CD19 T cell that secretes a CD19-anti-Her2 bridging protein. This cell therapy strategy exploits the ability of CD19-targeting CAR T cells to interact with CD19 on normal B cells to drive expansion, persistence and fitness. The secreted bridging protein potently binds to Her2-positive tumor cells, mediating CAR-CD19 T cell cytotoxicity in vitro and in vivo. Because of its short half-life, the secreted bridging protein will selectively accumulate at the site of highest antigen expression, ie. at the tumor. Bridging proteins that bind to multiple different tumor antigens have been created. Therefore, antigen-bridging CAR-CD19 T cells incorporate critical attributes for successful solid tumor cell therapy. This platform can be exploited to attack tumor antigens on any cancer.
Conflict of interest statement
The authors have read the journal’s policy, and the authors of the study have the following competing interests to declare: Aleta Biotherapeutics is wholly funded by Advent Life Science Partners, a venture capital firm. Advent Life Sciences provided support in the form of salaries for authors [CA, LS, LW, FJD, AB, PDR], and paid consulting fees [RRL]. Aleta Biotherapeutics funded BJH’s research at the University of Minnesota for a period of time, resulting in a shared patent filing. The agreement with Univ Minnesota was for a one-time payment from Aleta to secure all of the patent rights (assigned by Dr Hackel to UMN). The patent is "CD19 VARIANTS" US Appln. No. 62/599,211; Filed: December 15, 2017. The research sponsorship has since ended and that financial relationship in no manner has influenced the work contained in this manuscript. Aleta Biotherapeutics paid consultancy fees to HA in the past. The consultancy has since ended and that financial relationship in no manner has influenced the work contained in this manuscript. This does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no other products in development or marketed products associated with this research to declare.
Figures

Similar articles
-
Evaluation of Nonviral piggyBac and lentiviral Vector in Functions of CD19chimeric Antigen Receptor T Cells and Their Antitumor Activity for CD19+ Tumor Cells.Front Immunol. 2022 Jan 10;12:802705. doi: 10.3389/fimmu.2021.802705. eCollection 2021. Front Immunol. 2022. PMID: 35082789 Free PMC article.
-
Dual Effects of Cyclooxygenase Inhibitors in Combination With CD19.CAR-T Cell Immunotherapy.Front Immunol. 2021 May 26;12:670088. doi: 10.3389/fimmu.2021.670088. eCollection 2021. Front Immunol. 2021. PMID: 34122428 Free PMC article.
-
Preclinical Assessment of Efficacy and Safety Analysis of CAR-T Cells (ISIKOK-19) Targeting CD19-Expressing B-Cells for the First Turkish Academic Clinical Trial with Relapsed/Refractory ALL and NHL Patients.Turk J Haematol. 2020 Nov 19;37(4):234-247. doi: 10.4274/tjh.galenos.2020.2020.0070. Epub 2020 Aug 4. Turk J Haematol. 2020. PMID: 32755128 Free PMC article.
-
Open access? Widening access to chimeric antigen receptor (CAR) therapy for ALL.Exp Hematol. 2018 Oct;66:5-16. doi: 10.1016/j.exphem.2018.07.002. Epub 2018 Jul 20. Exp Hematol. 2018. PMID: 30031849 Review.
-
Chimeric Antigen Receptor T Cell Therapy for Pediatric B-ALL: Narrowing the Gap Between Early and Long-Term Outcomes.Front Immunol. 2020 Aug 11;11:1985. doi: 10.3389/fimmu.2020.01985. eCollection 2020. Front Immunol. 2020. PMID: 32849662 Free PMC article. Review.
Cited by
-
Anti-CD19 CAR T Cells That Secrete a Biparatopic Anti-CLEC12A Bridging Protein Have Potent Activity Against Highly Aggressive Acute Myeloid Leukemia In Vitro and In Vivo.Mol Cancer Ther. 2021 Oct;20(10):2071-2081. doi: 10.1158/1535-7163.MCT-20-1030. Epub 2021 Jul 12. Mol Cancer Ther. 2021. PMID: 34253594 Free PMC article.
-
Next-generation chimeric antigen receptors for T- and natural killer-cell therapies against cancer.Immunol Rev. 2023 Nov;320(1):217-235. doi: 10.1111/imr.13255. Epub 2023 Aug 7. Immunol Rev. 2023. PMID: 37548050 Free PMC article. Review.
-
Discovery and engineering of AiEvo2, a novel Cas12a nuclease for human gene editing applications.J Biol Chem. 2024 Mar;300(3):105685. doi: 10.1016/j.jbc.2024.105685. Epub 2024 Jan 23. J Biol Chem. 2024. PMID: 38272227 Free PMC article.
-
TIM-3, LAG-3, or 2B4 gene disruptions increase the anti-tumor response of engineered T cells.Front Immunol. 2024 Feb 29;15:1315283. doi: 10.3389/fimmu.2024.1315283. eCollection 2024. Front Immunol. 2024. PMID: 38510235 Free PMC article.
-
Bio-functional hydrogel coated membranes to decrease T-cell exhaustion in manufacturing of CAR T-cells.Front Immunol. 2025 Jun 27;16:1513148. doi: 10.3389/fimmu.2025.1513148. eCollection 2025. Front Immunol. 2025. PMID: 40655138 Free PMC article.
References
-
- O’Leary MC, Lu X, Huang Y, Lin X, Mahmood I, Przepiorka D, et al.. FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-cell Precursor Acute Lymphoblastic Leukemia. Clin Cancer Res. 2019;25(4):1142–6. Epub 2018/10/13. 10.1158/1078-0432.CCR-18-2035 . - DOI - PubMed
-
- Alabanza L, Pegues M, Geldres C, Shi V, Wiltzius JJW, Sievers SA, et al.. Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains. Mol Ther. 2017;25(11):2452–65. Epub 2017/08/16. 10.1016/j.ymthe.2017.07.013 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

