The granuloma in cryptococcal disease
- PMID: 33735307
- PMCID: PMC7971563
- DOI: 10.1371/journal.ppat.1009342
The granuloma in cryptococcal disease
Abstract
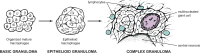
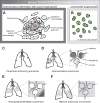
Although we have recognized cryptococcosis as a disease entity for well over 100 years, there are many details about its pathogenesis which remain unknown. A major barrier to better understanding is the very broad range of clinical and pathological forms cryptococcal infections can take. One such form has been historically called the cryptococcal granuloma, or the cryptococcoma. These words have been used to describe essentially any mass lesion associated with infection, due to their presumed similarity to the quintessential granuloma, the tubercle in tuberculosis. Although clear distinctions between tuberculosis and cryptococcal disease have been discovered, cellular and molecular studies still confirm some important parallels between these 2 diseases and what we now call granulomatous inflammation. In this review, we shall sketch out some of the history behind the term "granuloma" as it pertains to cryptococcal disease, explore our current understanding of the biology of granuloma formation, and try to place that understanding in the context of the myriad pathological presentations of this infection. Finally, we shall summarize the role of the granuloma in cryptococcal latency and present opportunities for future investigations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Stoddard JL, Cutler EC. Torula Infection in Man. New York: Rockefeller Institute for Medical Research; 1910 1915.
-
- Cox LB, Tolhurst JC. Human Torulosis. Melbourne and London: Melbourne University Press; 1946. 149 p.
-
- Mitchell DH, Sorrell TC, Allworth AM, Heath CH, McGregor AR, Papanaoum K, et al. Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin Infect Dis. 1995;20(3):611–6. Epub 1995/03/01. 10.1093/clinids/20.3.611 . - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

