Adenovirus-vectored T cell vaccine for hepacivirus shows reduced effectiveness against a CD8 T cell escape variant in rats
- PMID: 33735321
- PMCID: PMC8009437
- DOI: 10.1371/journal.ppat.1009391
Adenovirus-vectored T cell vaccine for hepacivirus shows reduced effectiveness against a CD8 T cell escape variant in rats
Abstract
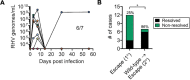
There is an urgent need for a vaccine to prevent chronic infection by hepatitis C virus (HCV) and its many genetic variants. The first human vaccine trial, using recombinant viral vectors that stimulate pan-genotypic T cell responses against HCV non-structural proteins, failed to demonstrate efficacy despite significant preclinical promise. Understanding the factors that govern HCV T cell vaccine success is necessary for design of improved immunization strategies. Using a rat model of chronic rodent hepacivirus (RHV) infection, we assessed the impact of antigenic variation and immune escape upon success of a conceptually analogous RHV T cell vaccine. Naïve Lewis rats were vaccinated with a recombinant human adenovirus expressing RHV non-structural proteins (NS)3-5B and later challenged with a viral variant containing immune escape mutations within major histocompatibility complex (MHC) class I-restricted epitopes (escape virus). Whereas 7 of 11 (64%) rats cleared infection caused by wild-type RHV, only 3 of 12 (25%) were protected against heterologous challenge with escape virus. Uncontrolled replication of escape virus was associated with durable CD8 T cell responses targeting escaped epitopes alone. In contrast, clearance of escape virus correlated with CD4 T cell helper immunity and maintenance of CD8 T cell responses against intact viral epitopes. Interestingly, clearance of wild-type RHV infection after vaccination conferred enhanced protection against secondary challenge with escape virus. These results demonstrate that the efficacy of an RHV T cell vaccine is reduced when challenge virus contains escape mutations within MHC class I-restricted epitopes and that failure to sustain CD8 T cell responses against intact epitopes likely underlies immune failure in this setting. Further investigation of the immune responses that yield protection against diverse RHV challenges in this model may facilitate design of broadly effective HCV vaccines.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Protection of Novel Adenovirus Vectored Vaccine in Rats Against Wild-Type Hepacivirus and Variant Infections.Liver Int. 2025 Apr;45(4):e70045. doi: 10.1111/liv.70045. Liver Int. 2025. PMID: 40095396
-
Use of an Outbred Rat Hepacivirus Challenge Model for Design and Evaluation of Efficacy of Different Immunization Strategies for Hepatitis C Virus.Hepatology. 2020 Mar;71(3):794-807. doi: 10.1002/hep.30894. Epub 2019 Oct 11. Hepatology. 2020. PMID: 31400152 Free PMC article.
-
Priming of Antiviral CD8 T Cells without Effector Function by a Persistently Replicating Hepatitis C-Like Virus.J Virol. 2020 May 4;94(10):e00035-20. doi: 10.1128/JVI.00035-20. Print 2020 May 4. J Virol. 2020. PMID: 32102885 Free PMC article.
-
T cell responses in hepatitis C virus infection: historical overview and goals for future research.Antiviral Res. 2015 Feb;114:96-105. doi: 10.1016/j.antiviral.2014.11.009. Epub 2014 Nov 26. Antiviral Res. 2015. PMID: 25433310 Free PMC article. Review.
-
Hepatitis C virus--T-cell responses and viral escape mutations.Eur J Immunol. 2012 Jan;42(1):17-26. doi: 10.1002/eji.201141593. Epub 2011 Nov 28. Eur J Immunol. 2012. PMID: 22125159 Review.
Cited by
-
Phenotype and fate of liver-resident CD8 T cells during acute and chronic hepacivirus infection.PLoS Pathog. 2023 Oct 9;19(10):e1011697. doi: 10.1371/journal.ppat.1011697. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37812637 Free PMC article.
-
Developing T Cell Epitope-Based Vaccines Against Infection: Challenging but Worthwhile.Vaccines (Basel). 2025 Jan 28;13(2):135. doi: 10.3390/vaccines13020135. Vaccines (Basel). 2025. PMID: 40006681 Free PMC article. Review.
-
Design and nonviral delivery of live attenuated vaccine to prevent chronic hepatitis C virus-like infection.Nat Commun. 2025 Aug 15;16(1):7629. doi: 10.1038/s41467-025-62813-8. Nat Commun. 2025. PMID: 40817105 Free PMC article.
References
-
- Bartenschlager R, Baumert TF, Bukh J, Houghton M, Lemon SM, Lindenbach BD, et al.. Critical challenges and emerging opportunities in hepatitis C virus research in an era of potent antiviral therapy: Considerations for scientists and funding agencies. Virus Res. 2018;248:53–62. 10.1016/j.virusres.2018.02.016 - DOI - PubMed
-
- Rossi C, Butt ZA, Wong S, Buxton J, Islam N, Yu A, et al.. Hepatitis C Virus Reinfection after Successful Treatment with Direct-Acting Antiviral Therapy in a Large Population-Based Cohort. Hepatology. 2018;68:907a–a. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

