Uncoupling Protein 2 Deficiency Enhances NLRP3 Inflammasome Activation Following Hyperglycemia-Induced Exacerbation of Cerebral Ischemia and Reperfusion Damage In Vitro and In Vivo
- PMID: 33735403
- PMCID: PMC8084809
- DOI: 10.1007/s11064-021-03270-9
Uncoupling Protein 2 Deficiency Enhances NLRP3 Inflammasome Activation Following Hyperglycemia-Induced Exacerbation of Cerebral Ischemia and Reperfusion Damage In Vitro and In Vivo
Abstract
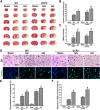
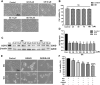
Mitochondrial uncoupling protein 2 (UCP2) deficiency exacerbates brain damage following cerebral ischemia/reperfusion (I/R). The Nod-like receptor protein-3 (NLRP3) inflammasome also plays a vital role in cerebral I/R damage. However, the effect of UCP2 on NLRP3 inflammasome-mediated hyperglycemia and I/R damage is not clear. In the present study, UCP2-knockout (UCP2-/-) and wild-type (WT) mice were used to establish a model of middle cerebral artery occlusion (MCAO) and reperfusion under normo- and hyperglycemic conditions. HT22 cells were established as a model of oxygen-glucose deprivation and reoxygenation (OGD/R) with high glucose to mimic hyperglycemia and I/R in vitro. HT22 cells were treated with/without different concentrations of the UCP2-specific inhibitor genipin for different periods of time. The results showed that UCP2 deficiency significantly increased histopathological changes and apoptosis after cerebral I/R damage in hyperglycemic mice. Moreover, UCP2 deficiency enhanced NLRP3 inflammasome activation in neurons when cerebral I/R damage was exacerbated by hyperglycemia. Furthermore, UCP2 deficiency enhanced NLRP3 inflammasome activation and reactive oxygen species (ROS) production in HT22 cells under OGD/R and high-glucose conditions. UCP2 deficiency aggravated hyperglycemia-induced exacerbation of cerebral I/R damage. UCP2 deficiency also enhanced NLRP3 inflammasome activation and ROS production in neurons in vitro and in vivo. These findings suggest that UCP2 deficiency enhances NLRP3 inflammasome activation following hyperglycemia-induced exacerbation of cerebral I/R damage in vitro and in vivo. UCP2 may be a potential therapeutic target for hyperglycemia-induced exacerbation of cerebral I/R damage.
Keywords: Cerebral ischemia; Hyperglycemia; Nod-like receptor protein-3; Reactive oxygen species; Uncoupling protein 2.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




Similar articles
-
Deletion of mitochondrial uncoupling protein 2 exacerbates mitophagy and cell apoptosis after cerebral ischemia and reperfusion injury in mice.Int J Med Sci. 2020 Oct 16;17(17):2869-2878. doi: 10.7150/ijms.49849. eCollection 2020. Int J Med Sci. 2020. PMID: 33162815 Free PMC article.
-
ALDH2 Attenuates Blood-Brain Barrier Injury Induced by Cerebral Ischemia/Reperfusion via Alleviating ROS/NLRP3 Inflammasome Axis.Neurochem Res. 2025 Jul 3;50(4):224. doi: 10.1007/s11064-025-04472-1. Neurochem Res. 2025. PMID: 40608173
-
Mitochondrial dysfunction induces NLRP3 inflammasome activation during cerebral ischemia/reperfusion injury.J Neuroinflammation. 2018 Aug 28;15(1):242. doi: 10.1186/s12974-018-1282-6. J Neuroinflammation. 2018. PMID: 30153825 Free PMC article.
-
Adiponectin peptide alleviates oxidative stress and NLRP3 inflammasome activation after cerebral ischemia-reperfusion injury by regulating AMPK/GSK-3β.Exp Neurol. 2020 Jul;329:113302. doi: 10.1016/j.expneurol.2020.113302. Epub 2020 Apr 8. Exp Neurol. 2020. PMID: 32275928 Review.
-
ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury.Oxid Med Cell Longev. 2016;2016:2183026. doi: 10.1155/2016/2183026. Epub 2016 Apr 5. Oxid Med Cell Longev. 2016. PMID: 27127546 Free PMC article. Review.
Cited by
-
The neuroprotective mechanism of lithium after ischaemic stroke.Commun Biol. 2022 Feb 3;5(1):105. doi: 10.1038/s42003-022-03051-2. Commun Biol. 2022. PMID: 35115638 Free PMC article.
-
Mitochondrial Quality Control in Health and Disease.MedComm (2020). 2025 Aug 15;6(8):e70319. doi: 10.1002/mco2.70319. eCollection 2025 Aug. MedComm (2020). 2025. PMID: 40821693 Free PMC article. Review.
-
NLRP3: Role in ischemia/reperfusion injuries.Front Immunol. 2022 Sep 27;13:926895. doi: 10.3389/fimmu.2022.926895. eCollection 2022. Front Immunol. 2022. PMID: 36238294 Free PMC article. Review.
-
Importance of Mitochondria in Cardiac Pathologies: Focus on Uncoupling Proteins and Monoamine Oxidases.Int J Mol Sci. 2023 Mar 30;24(7):6459. doi: 10.3390/ijms24076459. Int J Mol Sci. 2023. PMID: 37047436 Free PMC article. Review.
-
UCP2 inhibition exaggerates diabetic cardiomyopathy by facilitating the activation of NLRP3 and pyroptosis.Diabetol Metab Syndr. 2025 Jul 16;17(1):267. doi: 10.1186/s13098-025-01855-w. Diabetol Metab Syndr. 2025. PMID: 40671041 Free PMC article.
References
-
- Zhao J, Piao X, Wu Y, Liang SS, Han F, Liang Q, Shao SJ, Zhao DW. Cepharanthine attenuates cerebral ischemia and reperfusion injury by reducing NLRP3 inflammasome-induced inflammation and oxidative stress via inhibiting 12/15-LOX signaling. Biomed Pharmacother. 2020;127:110151. doi: 10.1016/j.biopha.2020.110151. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

