In vitro validation and characterization of pulsed inhaled nitric oxide administration during early inspiration
- PMID: 33735405
- PMCID: PMC7970749
- DOI: 10.1007/s10877-021-00689-x
In vitro validation and characterization of pulsed inhaled nitric oxide administration during early inspiration
Abstract
Purpose: Admixture of nitric oxide (NO) to the gas inspired with mechanical ventilation can be achieved through continuous, timed, or pulsed injection of NO into the inspiratory limb. The dose and timing of NO injection govern the inspired and intrapulmonary effect site concentrations achieved with different administration modes. Here we test the effectiveness and target reliability of a new mode injecting pulsed NO boluses exclusively during early inspiration.
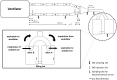
Methods: An in vitro lung model was operated under various ventilator settings. Admixture of NO through injection into the inspiratory limb was timed either (i) selectively during early inspiration ("pulsed delivery"), or as customary, (ii) during inspiratory time or (iii) the entire respiratory cycle. Set NO target concentrations of 5-40 parts per million (ppm) were tested for agreement with the yield NO concentrations measured at various sites in the inspiratory limb, to assess the effectiveness of these NO administration modes.
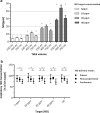
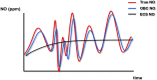
Results: Pulsed delivery produced inspiratory NO concentrations comparable with those of customary modes of NO administration. At low (450 ml) and ultra-low (230 ml) tidal volumes, pulsed delivery yielded better agreement of the set target (up to 40 ppm) and inspiratory NO concentrations as compared to customary modes. Pulsed delivery with NO injection close to the artificial lung yielded higher intrapulmonary NO concentrations than with NO injection close to the ventilator. The maximum inspiratory NO concentration observed in the trachea (68 ± 30 ppm) occurred with pulsed delivery at a set target of 40 ppm.
Conclusion: Pulsed early inspiratory phase NO injection is as effective as continuous or non-selective admixture of NO to inspired gas and may confer improved target reliability, especially at low, lung protective tidal volumes.
Keywords: ARDS; Artificial lung; Inhalation; Mechanical ventilation; Nitric oxide; PiNO.
© 2021. The Author(s).
Conflict of interest statement
Maria Deja has received a restricted grant from Linde® Gas Therapeutics, manufacturer and distributor of nitric oxide. Moritz Hofferberth has received salary in parts from this grant. Philipp Pickerodt has received one speaker fee from Linde® Gas Therapeutics. Rainer Köbrich is an employee of EKU Elektronik GmbH, the manufacturer of the NO Device used for this study. The authors declare that they have no further conflict of interests.
Figures
References
-
- Higenbottam TW, Pepke-Zaba J, Woolman P, Wallwork J. Inhaled endothelium-derived relaxing factor (EDRF) in primary pulmonary hypertension (PPH) Am Rev Respir Dis. 1988;137:A107.
-
- Lewandowski K. Nitric oxide as an adjunct. In: Tobin MJ, editor. Principles and practice of mechanical ventilation. New York: McGraw Hill; 2013. pp. 1389–1403.
-
- Afshari A, Brok J, Moller AM, Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome and acute lung injury in adults and children: a systematic review with meta-analysis and trial sequential analysis. Anesth Analg. 2011;211:1411–1421. doi: 10.1213/ANE.0b013e31820bd185. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

