Exercise for cancer cachexia in adults
- PMID: 33735441
- PMCID: PMC8094916
- DOI: 10.1002/14651858.CD010804.pub3
Exercise for cancer cachexia in adults
Abstract
Background: Cancer cachexia is a multifactorial syndrome characterised by an ongoing loss of skeletal muscle mass, with or without a loss of fat mass, leading to progressive functional impairment. Physical exercise may attenuate cancer cachexia and its impact on patient function. This is the first update of an original Cochrane Review published in Issue 11, 2014, which found no studies to include.
Objectives: To determine the effectiveness, acceptability and safety of exercise, compared with usual care, no treatment or active control, for cancer cachexia in adults.
Search methods: We searched CENTRAL, MEDLINE, Embase, and eight other databases to March 2020. We searched for ongoing studies in trial registries, checked reference lists and contacted experts to seek relevant studies.
Selection criteria: We sought randomised controlled trials in adults with cancer cachexia, that compared a programme of exercise alone or in combination with another intervention, with usual care, no treatment or an active control group.
Data collection and analysis: Two review authors independently assessed titles and abstracts for relevance and extracted data on study design, participants, interventions and outcomes from potentially relevant articles. We used standard methodological procedures expected by Cochrane. Our primary outcome was lean body mass and secondary outcomes were adherence to exercise programme, adverse events, muscle strength and endurance, exercise capacity, fatigue and health-related quality of life. We assessed the certainty of evidence using GRADE and included two Summary of findings tables.
Main results: We included four new studies in this update which overall randomised 178 adults with a mean age of 58 (standard deviation (SD) 8.2) years. Study sample size ranged from 20 to 60 participants and in three studies the proportion of men ranged from 52% to 82% (the fourth study was only available in abstract form). Three studies were from Europe: one in the UK and Norway; one in Belgium and one in Germany. The remaining study was in Canada. The types of primary cancer were head and neck (two studies), lung and pancreas (one study), and mixed (one study). We found two comparisons: exercise alone (strength-based exercise) compared to usual care (one study; 20 participants); and exercise (strength-based exercise/endurance exercise) as a component of a multimodal intervention (pharmacological, nutritional or educational (or a combination) interventions) compared with usual care (three studies, 158 participants). Studies had unclear and high risk of bias for most domains. Exercise plus usual care compared with usual care We found one study (20 participants). There was no clear evidence of a difference for lean body mass (8 weeks: MD 6.40 kg, 95% CI -2.30 to 15.10; very low-certainty evidence). For our secondary outcomes, all participants adhered to the exercise programme and no participant reported any adverse event during the study. There were no data for muscle strength and endurance, or maximal and submaximal exercise capacity. There was no clear evidence of a difference for either fatigue (4 to 20 scale, lower score was better) (8 weeks: MD -0.10, 95% CI -4.00 to 3.80; very low-certainty evidence) or health-related quality of life (0 to 104 scale, higher score was better) (8 weeks: MD 4.90, 95% CI -15.10 to 24.90; very low-certainty evidence). Multimodal intervention (exercise plus other interventions) plus usual care compared with usual care We found three studies but outcome data were only available for two studies. There was no clear evidence of a difference for lean body mass (6 weeks: MD 7.89 kg, 95% CI -9.57 to 25.35; 1 study, 44 participants; very low-certainty evidence; 12 weeks: MD -2.00, 95% CI -8.00 to 4.00; one study, 60 participants; very low-certainty evidence). For our secondary outcomes, there were no data reported on adherence to the exercise programme, endurance, or maximal exercise capacity. In one study (44 participants) there was no clear evidence of a difference for adverse events (patient episode report) (6 weeks: risk ratio (RR) 1.18, 95% CI 0.67 to 2.07; very low-certainty evidence). Another study assessed adverse events but reported no data and the third study did not assess this outcome. There was no clear evidence of a difference in muscle strength (6 weeks: MD 3.80 kg, 95% CI -2.87 to 10.47; 1 study, 44 participants; very low-certainty evidence; 12 weeks MD -5.00 kg, 95% CI -14.00 to 4.00; 1 study, 60 participants; very low-certainty evidence), submaximal exercise capacity (6 weeks: MD -16.10 m walked, 95% CI -76.53 to 44.33; 1 study, 44 participants; very low-certainty evidence; 12 weeks: MD -62.60 m walked, 95% CI -145.87 to 20.67; 1 study, 60 participants; very low-certainty evidence), fatigue (0 to 10 scale, lower score better) (6 weeks: MD 0.12, 95% CI -1.00 to 1.24; 1 study, 44 participants; very low-certainty evidence) or health-related quality of life (0 to 104 scale, higher score better) (12 weeks: MD -2.20, 95% CI -13.99 to 9.59; 1 study, 60 participants; very low-certainty evidence).
Authors' conclusions: The previous review identified no studies. For this update, our conclusions have changed with the inclusion of four studies. However, we are uncertain of the effectiveness, acceptability and safety of exercise for adults with cancer cachexia. Further high-quality randomised controlled trials are still required to test exercise alone or as part of a multimodal intervention to improve people's well-being throughout all phases of cancer care. We assessed the certainty of the body of evidence as very low, downgraded due to serious study limitations, imprecision and indirectness. We have very little confidence in the results and the true effect is likely to be substantially different from these. The findings of at least three more studies (one awaiting classification and two ongoing) are expected in the next review update.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
AJG: none.
VS: none.
LSN: none.
JPTB: none.
MSP: none.
MM: received personal fees for lectures and consultancy with Chugai UK (2015), Helsinn (2015 to 2017) and Fresenius Kabi (2016 to 2019) relating to cancer cachexia.
Figures
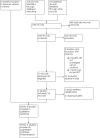










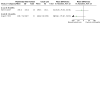


Update of
-
Exercise for cancer cachexia in adults.Cochrane Database Syst Rev. 2014 Nov 26;(11):CD010804. doi: 10.1002/14651858.CD010804.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2021 Mar 18;3:CD010804. doi: 10.1002/14651858.CD010804.pub3. PMID: 25424884 Updated.
Comment in
-
Is Exercise Effective and Safe for Cancer Cachexia in Adults?: A Cochrane Review Summary With Commentary.Am J Phys Med Rehabil. 2022 Aug 1;101(8):795-797. doi: 10.1097/PHM.0000000000001973. Epub 2022 Jan 21. Am J Phys Med Rehabil. 2022. PMID: 35067553 No abstract available.
References
References to studies included in this review
Capozzi 2016 {published data only}
-
- Capozzi LC, McNeely ML, Lau HY, Reimer RA, Giese-Davis J, Fung TS, et al. Patient-reported outcomes, body composition, and nutrition status in patients with head and neck cancer: results from an exploratory randomized controlled exercise trial. Cancer 2016;122(8):1185-200. - PubMed
-
- NCT01681654. Exercise and Nutrition for Head And Neck Cancer Patients (ENHANCE). clinicaltrials.gov/show/NCT01681654 (first received 10 September 2012).
Forget 2014 {published data only}
-
- Forget F, Frusch N, Trokay L, Archen C, Courtois a-C. A randomized trial comparing best supportive care (BSC) versus multimodality approach (MA) to fight against cachexia in patients with cancer treated with chemotherapy. Journal of Clinical Oncology 2014;32(15 Suppl 1):e20655. [DOI: 10.1200/jco.2014.32.15_suppl.e20655 ]
Grote 2018 {published data only}
-
- NCT03524755. Physical exercise for patients who suffer from weight loss due to head and neck cancer undergoing medical treatment. clinicaltrials.gov/show/NCT03524755 (first received 15 May 2018).
Solheim 2017 {published data only}
-
- EudraCT 2010-022897-14. PreMENAC: multimodal exercise/nutrition/anti-inflammatory treatment for cachexia: a feasibility study (phase II). www.clinicaltrialsregister.eu/ctr-search/trial/2010-022897-14/results (first received 18 September 2016).
-
- Kaasa S, Solheim T, Laird BJ, Balstad T, Stene GB, Bye A, et al. A randomised, open-label trial of a multimodal intervention (exercise, nutrition and anti-inflammatory medication) plus standard care versus standard care alone to prevent/attenuate cachexia in advanced cancer patients undergoing chemotherapy. Journal of Clinical Oncology 2015;33(15 Suppl):9628.
-
- NCT01419145. A feasibility study of multimodal exercise/nutrition/anti-inflammatory treatment for cachexia – the pre-MENAC Study. clinicaltrials.gov/show/NCT01419145 (first received 17 August 2011).
-
- Rakel Balstad T, Solheim TS, Laird B, Fearon K, Kaasa S, Bye A. PP090-SUN: Feasibility of dietary counseling to attenuate cachexia in patients with advanced cancer. Clinical Nutrition 2015;33:S53.
References to studies excluded from this review
Arrieta 2019 {published data only}
Battaglini 2010 {published data only}
-
- Battaglini C, Groff D, Shields E, Evans E, Naumann F, Peppercorn J, et al. Exercise and psychosocial interventions in breast cancer survivors: preliminary results of a randomized controlled trial. Medicine and Science in Sports and Exercise 2010;42(5):344-5.
Carnaby‐Mann 2012 {published data only}
-
- Carnaby-Mann G, Crary MA, Schmalfuss I, Amdur R. "Pharyngocise": randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. International Journal of Radiation Oncology, Biology, Physics 2012;83(1):210-9. - PubMed
Cheville 2010 {published data only}
-
- Cheville AL, Girardi J, Clark MM, Rummans TA, Pittelkow T, Brown P, et al. Therapeutic exercise during out patient radiation therapy for advanced cancer: feasibility and impact on physical well-being. American Journal of Physical Medicine and Rehabilitation 2010;89(8):611-9. - PubMed
Courneya 2009 {published data only}
-
- Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. Journal of Clinical Oncology 2009;27(27):4605-12. - PubMed
Elter 2009 {published data only}
-
- Elter T, Stipanov M, Heuser E, Bergwelt-Baildon M, Bloch W, Hallek M, et al. Is physical exercise possible in patients with critical cytopenia undergoing intensive chemotherapy for acute leukaemia or aggressive lymphoma? International Journal of Hematology 2009;90(2):199-204. - PubMed
Fouladiun 2007 {published data only}
-
- Fouladiun M, Körner U, Gunnebo L, Sixt-Ammilon P, Bosaeus I, Lundholm K. Daily physical-rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clinical Cancer Research 2007;13(21):6379-85. - PubMed
Irwin 2009 {published data only}
Jain 2019 {published data only}
Kuehr 2014 {published data only}
-
- Kuehr L, Wiskemann J, Abel U, Ulrich CM, Hummler S, Thomas M. Exercise inpatients with non-small cell lung cancer. Medicine and Science in Sports and Exercise 2014;46(4):656-63. - PubMed
Litterini 2013 {published data only}
-
- Litterini AJ, Fieler VK, Cavanaugh JT, Lee JQ. Differential effects of cardiovascular and resistance exercise on functional mobility in individuals with advanced cancer: a randomized trial. Archives of Physical Medicine and Rehabilitation 2013;94(12):2329-35. - PubMed
Mantovani 2010 {published data only}
-
- Mantovani G, Macciò A, Madeddu C, Dessì M, Serpe R, Antoni G, et al. Focus on the assessment of physical activity level of patients with cancer cachexia enrolled in a randomized phase III clinical trial. Journal of Clinical Oncology 2010;28(15):9164.
-
- Mantovani G. Randomised phase III clinical trial of 5 different arms of treatment on 332 patients with cancer cachexia. European Review for Medical and Pharmacological Sciences 2010;14(4):292-301. - PubMed
Oldervoll 2006 {published data only}
-
- Oldervoll LM, Loge JH, Paltiel H, Asp MB, Vidvei U, Wiken AN, et al. The effect of a physical exercise program in palliative care: a phase II study. Journal of Pain and Symptom Management 2006;31(5):421-30. - PubMed
Oldervoll 2011 {published data only}
Op den Kamp 2012 {published data only}
-
- Op den Kamp CM, Langen RC, Minnaard R, Kelders MC, Snepvangers FJ, Hesselink MK, et al. Pre-cachexia in patients with stages I-III non-small cell lung cancer: systemic inflammation and functional impairment without activation of skeletal muscle ubiquitin proteasome system. Lung Cancer 2012;76(1):112-7. [DOI: 10.1016/j.lungcan.2011.09.012] - DOI - PubMed
Saarto 2012 {published data only}
-
- Saarto T. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporosis International 2012;23(5):1601-12. - PubMed
Schwartz 2007 {published data only}
-
- Schwartz AL, Winters-Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncology Nursing Forum 2007;34(3):627-33. - PubMed
Solheim 2019 {published data only}
-
- Solheim TS, Vagnildhaug OM, Laird BJ, Balstad TR. Combining optimal nutrition and exercise in a multimodal approach for patients with active cancer and risk for losing weight: rationale and practical approach. Nutrition 2019;Nov-Dec:67-8. - PubMed
Uster 2018 {published data only}
Vanderbyl 2017 {published data only}
-
- Vanderbyl BL, Mayer MJ, Nash C, Tran AT, Windholz T, Swanson T, et al. A comparison of the effects of medical Qigong and standard exercise therapy on symptoms and quality of life in patients with advanced cancer. Supportive Care in Cancer 2017;25(6):1749-58. [DOI: 10.1007/s00520-017-3579-x] - DOI - PubMed
Zatarain 2013 {published data only}
-
- Zatarain LA. Body composition in head and neck cancer patients undergoing concurrent chemoradiation. Journal of Clinical Oncology 2013;31 Suppl 1:15.
References to studies awaiting assessment
Rogers 2011 {published data only}
-
- ACTRN12611000870954. Anti-inflammatory and nutritional support, with simple exercises in lung cancer patients with weight loss. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=343304 (first received 16 August 2011).
-
- Rogers ES, MacLeod RD, Stewart J, Bird SP, Keogh JW. A randomised feasibility study of EPA and COX-2 inhibitor (Celebrex) versus EPA, COX-2 inhibitor (Celebrex), resistance training followed by ingestion of essential amino acids high in leucine in NSCLC cachectic patients – ACCeRT study. BMC Cancer 2011;11:493. - PMC - PubMed
References to ongoing studies
ACTRN12619000426189 {published data only}
-
- ACTRN12619000426189. ACE trial: the Advanced cancer & Cachexia Exercise trial. mmihr.acu.edu.au/projects/ace-trial/ (accessed 31 July 2019).
-
- ACTRN12619000426189. Feasibility and efficacy of exercise in advanced cancer patients with cachexia. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12619000426189 (first received 19 February 2019).
Solheim 2018 {published data only}
-
- EudraCT 2013-002282-19. A randomised, open-label trial of a multimodal intervention (exercise, nutrition and anti-inflammatory medication) plus standard care versus standard care alone to prevent / attenuate cachexia in advanced cancer patients undergoing chemotherapy. www.clinicaltrialsregister.eu/ctr-search/trial/2013-002282-19/GB (first received 7 April 2016).
-
- MENAC-2013-05. A randomised, open-label trial of a multimodal intervention (exercise, nutrition and anti-inflammatory medication) plus standard care versus standard care alone to prevent/attenuate cachexia in advanced cancer patients undergoing chemotherapy. bonn.clinicalsite.org/de/cat/457/trial/3212 (accessed 7 August 2019).
-
- NCT02330926. Multimodal intervention for cachexia in advanced cancer patients undergoing chemotherapy (MENAC). clinicaltrials.gov/ct2/show/NCT02330926 (first received 5 January 2015).
-
- Solheim TS, Laird BJ, Balstad TR, Bye A, Stene G, Baracos V, et al. Cancer cachexia: rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Supportive Palliative Care 2018;8(3):258-65. - PubMed
Additional references
Antoun 2013
-
- Antoun S, Lanoy E, Iacovelli R, Albiges-Sauvin L, Loriot Y, Merad-Taoufik M, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 2013;25:2821-8. - PubMed
Arends 2017
-
- Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clinical Nutrition 2017;1:11-48. - PubMed
Argilés 2012
Baracos 2018
-
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KC. Cancer-associated cachexia. Nature Reviews Disease Primers 2018;4:17105. - PubMed
Betof 2013
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011 Available from training.cochrane.org/handbook/archive/v5.1/.
Dewey 2007
Dodson 2011
-
- Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annual Review of Medicine 2011;62:265-79. - PubMed
England 2012
-
- England R, Maddocks M, Manderson C, Wilcock A. Factors influencing exercise performance in thoracic cancer. Respiratory Medicine 2012;106:294-9. - PubMed
Evans 2008
Fearon 2008
-
- Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. European Journal of Cancer 2008;44(8):1124-32. - PubMed
Fearon 2011
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncology 2011;12(5):489-95. [PMID: ] - PubMed
Fearon 2012
-
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signalling and metabolic pathways. Cell Metabolism 2012;16:153-66. - PubMed
Gleeson 2011
-
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nature Reviews. Immunology 2011;9:607-15. - PubMed
Glover 2010
-
- Glover EI, Phillips SM. Resistance exercise and appropriate nutrition to counteract muscle wasting and promote muscle hypertrophy. Current Opinion in Clinical Nutrition and Metabolic Care 2010;13:630-4. - PubMed
Gould 2013
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 5 February 2021. Available at gradepro.org.
Guyatt 2008
Hall 2019
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-59. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2 (updated June 2017). Cochrane, 2017. training.cochrane.org/handbook/archive/v5.2.
Hoffmann 2014
-
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. - PubMed
Jones 2012
Laviano 2005
-
- Laviano A, Meguid MM, Inui A, Muscaritoli M, Rossi-Fanelli F. Therapy insight: cancer anorexia-cachexia syndrome – when all you can eat is yourself. Nature Clinical Practice Oncology 2005;2:159-65. - PubMed
LeBlanc 2015
-
- LeBlanc TW, Nipp RD, Rushing CN, Samsa GP, Locke SC, Kamal AH, et al. Correlation between the international consensus definition of the Cancer Anorexia-Cachexia Syndrome (CACS) and patient-centered outcomes in advanced non-small cell lung cancer. Journal of Pain and Symptom Management 2015;49(4):680-9. - PubMed
Lee 2011
Maddocks 2011
Maddocks 2012
-
- Maddocks M, Murton AJ, Wilcock A. Therapeutic exercise in cancer cachexia. Critical Reviews in Oncogenesis 2012;17:285-92. - PubMed
Marimuthu 2011
-
- Marimuthu K, Murton AJ, Greenhaff PL. Mechanisms regulating muscle mass during disuse atrophy and rehabilitation in humans. Journal of Applied Physiology 2011;110:555-60. - PubMed
Martin 2013
-
- Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. Journal of Clinical Oncology 2013;31(12):1539-47. - PubMed
McMillan 2013
-
- McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treatment Reviews 2013;39:534-40. - PubMed
Moses 2009
-
- Moses AG, Maingay J, Sangster K, Fearon KC, Ross JA. Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: relationship to acute phase response and survival. Oncology Reports 2009;21:1091-5. - PubMed
Muscaritoli 2010
-
- Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Specialist Interest Groups (SIG) 'cachexia – anorexia in chronic wasting diseases' and 'nutrition in genetics'. Clinical Nutrition 2010;29:154-9. - PubMed
Naito 2017
-
- Naito T, Okayama T, Aoyama T, Ohashi T, Masuda Y, Kimura M, et al. Unfavorable impact of cancer cachexia on activity of daily living and need for inpatient care in elderly patients with advanced non-small-cell lung cancer in Japan: a prospective longitudinal observational study. BMC Cancer 2017;17(1):800. - PMC - PubMed
Payne 2017
Prado 2007
-
- Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clinical Cancer Research 2007;13:3264-8. - PubMed
Prado 2008
-
- Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncology 2008;9:629-35. - PubMed
Prado 2009
-
- Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clinical Cancer Research 2009;15:2920-6. - PubMed
Proctor 2011
Radbruch 2010
-
- Radbruch L, Elsner F, Trottenberg P, Strasser F, Fearon K. Clinical Practise Guidelines on Cancer Cachexia in Advanced Cancer Patients. Aachen: Department of Palliative Medicine/European Palliative Care Research Collaborative, 2010.
Reid 2012
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roeland 2020
-
- Roeland EJ, Bohlke K, Baracos VE, Bruera E, Fabbro Ed, Dixon S, et al. Management of cancer cachexia: ASCO guideline. Journal of Clinical Oncology 2020;38(21):2438-53. - PubMed
Ruiz Garcia 2013
Schünemann 2019
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated August 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Solheim 2012
-
- Solheim TS, Laird BJ. Evidence base for multimodal therapy in cachexia. Current Opinion in Supportive and Palliative Care 2012;6(4):424-31. - PubMed
Starkie 2003
-
- Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNFα production in humans. FASEB Journal 2003;17:884-6. - PubMed
Stephens 2012
-
- Stephens NA, Gray C, MacDonald AJ, Tan BH, Gallagher IJ, Skipworth RJ, et al. Sexual dimorphism modulates the impact of cancer cachexia on lower limb muscle mass and function. Clinical Nutrition 2012;31:499-505. - PubMed
Thompson 2010
-
- Thompson WR, Gordon NF, Pescatello LS. ACSM's Guidelines for Exercise Testing and Prescription. 8th edition. Philadelphia (PA): Lippincott Williams & Wilkins, 2010.
Tisdale 2009
Wang 2006
-
- Wang X, Hu Z, J Hu, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006;147(9):4160-8. - PubMed
Weber 2009
-
- Weber MA, Krakowski-Roosen H, Schroder L, Kinscherf R, Krix M, Kopp-Schneider A, et al. Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer-related cachexia. Acta Oncologica 2009;48:116-24. - PubMed
Wilcock 2008
-
- Wilcock A, Maddocks M, Lewis M, England R, Manderson C. Symptoms limiting activity in cancer patients with breathlessness on exertion: ask about muscle fatigue. Thorax 2008;63:91-2. - PubMed
Wilms 2016
-
- Wilms B, Schmid SM, Luley K, Wiskemann J, Lehnert H. Prevention and treatment of cachexia: exercise and nutritional therapy. Der Internist 2016;57(10):971-7. - PubMed
References to other published versions of this review
Grande 2013
Grande 2014
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

