Retinal layer thinning predicts treatment failure in relapsing multiple sclerosis
- PMID: 33735479
- PMCID: PMC8251588
- DOI: 10.1111/ene.14829
Retinal layer thinning predicts treatment failure in relapsing multiple sclerosis
Abstract
Background and purpose: Peripapillary retinal nerve fiber layer (pRNFL) and macular ganglion cell plus inner plexiform layer (GCIPL) thinning are markers of neuroaxonal degeneration in multiple sclerosis (MS), which is reduced by disease-modifying treatment (DMT). We aimed to investigate the potential of pRNFL and GCIPL thinning for prediction of DMT failure in relapsing MS (RMS).
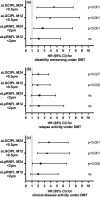
Methods: In this 4-year prospective observational study on 113 RMS patients, pRNFL and GCIPL were measured at DMT initiation and after 12 months (M12) and 24 months (M24). Treatment failure was defined as 6-month confirmed Expanded Disability Status Scale (EDSS) progression and/or Symbol Digit Modalities Test (SDMT) worsening. Optimal cutoff values for predicting treatment failure were determined by receiver operating characteristic analyses and hazard ratios (HRs) by multivariable Cox regression adjusting for age, sex, disease duration, EDSS/SDMT, and DMT class.
Results: Thinning of GCIPL >0.5 μm/year at M24 showed superior value for treatment failure prediction (HR: 4.5, 95% confidence interval [CI]: 1.8-7.6, p < 0.001; specificity 91%, sensitivity 81%), followed by GCIPL >0.5 μm at M12 (odds ratio [OR]: 3.9, 95% CI: 1.4-6.9, p < 0.001; specificity 85%, sensitivity 78%), and pRNFL ≥2 μm/year at M24 (OR: 3.7, 95% CI: 1.1-6.5, p = 0.023; specificity 84%, sensitivity 69%), whereas pRNFL at M12 was not predictive.
Conclusions: GCIPL, and to a lesser degree pRNFL, thinning predicts disability progression after DMT initiation and may be a useful and accessible biomarker of treatment failure in RMS.
Keywords: GCIPL; OCT; disease-modifying treatment; multiple sclerosis; retinal thinning.
© 2021 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
G.B. has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene, Merck, Novartis, Sanofi‐Genzyme, and Teva, and received honoraria for consulting from Biogen, Roche, and Teva. H.H. has participated in meetings sponsored by, received speaker honoraria or travel funding from Bayer, Biogen, Merck, Novartis, Sanofi‐Genzyme, Siemens, and Teva, and received honoraria for consulting from Biogen and Teva. P.A. has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Merck, Roche, Sanofi‐Genzyme, and Teva, and received honoraria for consulting from Biogen. He received a research grant from Quanterix International and was awarded a combined sponsorship from Biogen, Merck, Roche, Sanofi‐Genzyme, and Teva for a clinical study. M.A. received speaker honoraria and/or travel grants from Biogen, Merck, Novartis, and Sanofi‐Genzyme. K.B. has participated in meetings sponsored by and received travel funding from Roche. F.D.P. has participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations) or travel funding from Bayer, Biogen, Celgene, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva. F.L. has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Bayer, Biogen, Celgene, MedDay, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva. P.R. has received honoraria for consultancy/speaking from AbbVie, Alexion, Almirall, Biogen, Merck, Novartis, Roche, Sandoz, Sanofi‐Genzyme, and has received research grants from Amicus, Biogen, Merck, and Roche. S.W. has participated in meetings sponsored by, received honoraria or travel funding from Biogen, Merck, Novartis, Sanofi‐Genzyme, Teva, Allergan, Ipsen Pharma, and Roche. A.Z. has participated in meetings sponsored by, received speaking honoraria or travel funding from Biogen, Merck, Sanofi‐Genzyme, and Teva. T.Z. has participated in meetings sponsored by or received travel funding from Biogen, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva. F.D. has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Alexion, Almirall, Biogen, Celgene, Merck, Novartis, Roche, and Sanofi‐Genzyme. His institution received scientific grants from Biogen and Sanofi‐Genzyme. T.B. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Almirall, Bayer, Biogen, Biologix, Bionorica, Celgene, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi‐Genzyme, Teva, and TG Pharmaceuticals. His institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Merck, Novartis, Sanofi‐Genzyme, Teva) and for participation in clinical trials in MS sponsored by Alexion, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi‐Genzyme, and Teva.
Figures



Similar articles
-
Retinal thinning differentiates treatment effects in relapsing multiple sclerosis below the clinical threshold.Ann Clin Transl Neurol. 2025 Feb;12(2):345-354. doi: 10.1002/acn3.52279. Epub 2024 Dec 16. Ann Clin Transl Neurol. 2025. PMID: 39686570 Free PMC article.
-
Retinal layer thickness predicts disability accumulation in early relapsing multiple sclerosis.Eur J Neurol. 2023 Apr;30(4):1025-1034. doi: 10.1111/ene.15718. Epub 2023 Feb 16. Eur J Neurol. 2023. PMID: 36719184
-
Retinal layer thinning is reflecting disability progression independent of relapse activity in multiple sclerosis.Mult Scler J Exp Transl Clin. 2020 Oct 29;6(4):2055217320966344. doi: 10.1177/2055217320966344. eCollection 2020 Oct-Dec. Mult Scler J Exp Transl Clin. 2020. PMID: 33194221 Free PMC article.
-
The Effects of Disease-Modifying Therapies on Optic Nerve Degeneration in Multiple Sclerosis.Eur J Neurol. 2025 Mar;32(3):e70081. doi: 10.1111/ene.70081. Eur J Neurol. 2025. PMID: 40047132 Free PMC article. Review.
-
Retinal optical coherence tomography measures in multiple sclerosis: a systematic review and meta-analysis.Ann Clin Transl Neurol. 2024 Sep;11(9):2236-2253. doi: 10.1002/acn3.52165. Epub 2024 Jul 28. Ann Clin Transl Neurol. 2024. PMID: 39073308 Free PMC article.
Cited by
-
Optical coherence tomography assessment of axonal and neuronal damage of the retina in patients with familial and sporadic multiple sclerosis.Front Neurol. 2022 Sep 16;13:953188. doi: 10.3389/fneur.2022.953188. eCollection 2022. Front Neurol. 2022. PMID: 36188381 Free PMC article.
-
Effects of Ibudilast on Retinal Atrophy in Progressive Multiple Sclerosis Subtypes: Post Hoc Analyses of the SPRINT-MS Trial.Neurology. 2023 Sep 5;101(10):e1014-e1024. doi: 10.1212/WNL.0000000000207551. Epub 2023 Jul 17. Neurology. 2023. PMID: 37460235 Free PMC article. Clinical Trial.
-
Evolution of retinal degeneration and prediction of disease activity in relapsing and progressive multiple sclerosis.Nat Commun. 2024 Jun 19;15(1):5243. doi: 10.1038/s41467-024-49309-7. Nat Commun. 2024. PMID: 38897994 Free PMC article.
-
Retinal Optical Coherence Tomography Longitudinal Measures as Prognostic Biomarkers in Multiple Sclerosis: Systematic Review and Meta-Analysis.Neurol Neuroimmunol Neuroinflamm. 2025 Jul;12(4):e200416. doi: 10.1212/NXI.0000000000200416. Epub 2025 May 27. Neurol Neuroimmunol Neuroinflamm. 2025. PMID: 40424561 Free PMC article.
-
Emerging imaging and liquid biomarkers in multiple sclerosis.Eur J Immunol. 2023 Aug;53(8):e2250228. doi: 10.1002/eji.202250228. Epub 2023 May 28. Eur J Immunol. 2023. PMID: 37194443 Free PMC article. Review.
References
-
- Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221‐1231. - PubMed
-
- Cree BAC, Mares J, Hartung H‐P. Current therapeutic landscape in multiple sclerosis. Curr Opin Neurol. 2019;32:365‐377. - PubMed
-
- Giovannoni G. Disease‐modifying treatments for early and advanced multiple sclerosis. Curr Opin Neurol. 2018;31:1‐11. - PubMed
-
- Bsteh G, Hegen H, Dosser C, et al. To treat or not to treat – sequential individualized treatment evaluation in relapsing multiple sclerosis. Mult Scler Relat Dis. 2019;101908. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

