Dornase alfa for cystic fibrosis
- PMID: 33735508
- PMCID: PMC8094421
- DOI: 10.1002/14651858.CD001127.pub5
Dornase alfa for cystic fibrosis
Abstract
Background: Dornase alfa is currently used as a mucolytic to treat pulmonary disease (the major cause of morbidity and mortality) in cystic fibrosis. It reduces mucus viscosity in the lungs, promoting improved clearance of secretions. This is an update of a previously published review.
Objectives: To determine whether the use of dornase alfa in cystic fibrosis is associated with improved mortality and morbidity compared to placebo or other medications that improve airway clearance, and to identify any adverse events associated with its use.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register which comprises references identified from comprehensive electronic database searches, handsearching relevant journals and abstracts from conferences. Date of the most recent search of the Group's Cystic Fibrosis Register: 12 October 2020. Clinicaltrials.gov and the International Clinical Trials Registry Platform were also searched to identify unpublished or ongoing trials. Date of most recent search: 08 February 2021.
Selection criteria: All randomised and quasi-randomised controlled trials comparing dornase alfa to placebo, standard therapy or other medications that improve airway clearance.
Data collection and analysis: Authors independently assessed trials against the inclusion criteria; two authors carried out analysis of methodological quality and data extraction. GRADE was used to assess the level of evidence.
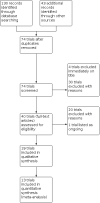
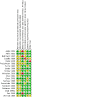
Main results: The searches identified 74 trials, of which 19 (2565 participants) met our inclusion criteria. 15 trials compared dornase alfa to placebo or no dornase alfa (2447 participants); two compared daily dornase to hypertonic saline (32 participants); one compared daily dornase alfa to hypertonic saline and alternate day dornase alfa (48 participants); one compared dornase alfa to mannitol and the combination of both drugs (38 participants). Trial duration varied from six days to three years. Dornase alfa compared to placebo or no treatment Dornase alfa probably improved forced expiratory volume at one second (FEV1) at one month (four trials, 248 participants), three months (one trial, 320 participants; moderate-quality evidence), six months (one trial, 647 participants; high-quality evidence) and two years (one trial, 410 participants). Limited low-quality evidence showed treatment may make little or no difference in quality of life. Dornase alfa probably reduced the number of pulmonary exacerbations in trials of up to two years (moderate-quality evidence). One trial that examined the cost of care, including the cost of dornase alfa, found that the cost savings from dornase alfa offset 18% to 38% of the medication costs. Dornase alfa: daily versus alternate day One cross-over trial (43 children) found little or no difference between treatment regimens for lung function, quality of life or pulmonary exacerbations (low-quality evidence). Dornase alfa compared to other medications that improve airway clearance Results for these comparisons were mixed. One trial (43 children) showed dornase alfa may lead to a greater improvement in FEV1 compared to hypertonic saline (low-quality evidence), and one trial (23 participants) reported little or no differences in lung function between dornase alfa and mannitol or dornase alfa and dornase alfa plus mannitol (low-quality evidence). One trial (23 participants) found dornase alfa may improve quality of life compared to dornase alfa plus mannitol (low-quality evidence); other comparisons found little or no difference in this outcome (low-quality evidence). No trials in any comparison reported any difference between groups in the number of pulmonary exacerbations (low-quality evidence). When all comparisons are assessed, dornase alfa did not cause significantly more adverse effects than other treatments, except voice alteration and rash.
Authors' conclusions: There is evidence to show that, compared with placebo, therapy with dornase alfa may improve lung function in people with cystic fibrosis in trials lasting from one month to two years. There was a decrease in pulmonary exacerbations in trials of six months or longer, probably due to treatment. Voice alteration and rash appear to be the only adverse events reported with increased frequency in randomised controlled trials. There is not enough evidence to firmly conclude if dornase alfa is superior to other hyperosmolar agents in improving lung function.
Trial registration: ClinicalTrials.gov NCT00557089 NCT00117208 NCT00446680 NCT00416182 NCT04320381 NCT01712334 NCT04378153.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Dr Connie Yang has no conflicts of interest.
Dr. Montgomery has no conflicts of interest.
Figures






















































Update of
-
Dornase alfa for cystic fibrosis.Cochrane Database Syst Rev. 2018 Sep 6;9(9):CD001127. doi: 10.1002/14651858.CD001127.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2021 Mar 18;3:CD001127. doi: 10.1002/14651858.CD001127.pub5. PMID: 30187450 Free PMC article. Updated.
References
References to studies included in this review
Adde 2004 {published data only}
-
- Adde FV, Borges KTL, Hatanaka ACF, Nakaie CMA, Cardieri JMA, Oliveira RC, et al. Hypertonic saline X recombinant human DNase: a randomised cross-over study in 18 cystic fibrosis patients. Journal of Cystic Fibrosis 2004;3(Suppl 1):S66. [CFGD REGISTER: BD113]
Amin 2011 {published data only}
-
- Amin R, Subbarao P, Lou W, Jabar A, Balkovec S, Jensen R, et al. The effect of dornase alfa on ventilation in homogeneity in patients with cystic fibrosis. European Respiratory Journal 2011;37(4):806-12. [CFGD REGISTER: BD163b] - PubMed
-
- Amin R, Subbarao P, Lou W, Jabar A, Balkovec S, Jensen R, et al. The effect of dornase alfa on ventilation in homogeneity in patients with cystic fibrosis. Pediatric Pulmonology 2010;45(Suppl 33):361. [ABSTRACT NO.: 396] [CFGD REGISTER: BD163a]
Ballmann 2002 {published data only}
-
- Ballmann M, Hardt H. Hypertonic saline and recombinant human DNase: a randomised cross-over pilot study in patients with cystic fibrosis. In: 22nd European Cystic Fibrosis Conference; 1998 June 13-19; Berlin, Germany. 1998:80. [CFGD REGISTER: BD70a] - PubMed
-
- Ballmann M, Hardt H. Hypertonic saline and recombinant human DNase: a randomised cross-over pilot study in patients with cystic fibrosis. Journal of Cystic Fibrosis 2002;1(1):35-7. [CFGD REGISTER: BD70b] - PubMed
Castile 2009 {published data only}
-
- Castile R, Mueller G, Long F, Flucke R, Baker B, Clifford B. Effects of nebulized recombinant human deoxyribonuclease (dornase alfa) in infants with CF evaluated using infant pulmonary function testing and high resolution computerized tomographic imaging of the chest. Pediatric Pulmonology 2009;32(Suppl 32):357. [ABSTRACT NO.: 414] [CFGD REGISTER: BD161a]
-
- NCT00179998. Efficacy of Pulmozyme in infants and young children with cystic fibrosis. clinicaltrials.gov/show/NCT00179998 (first posted 16 September 2005). [CFGD REGISTER: BD161b]
Dodd 2000 {published data only}
-
- Dodd ME, Moorcroft AJ, Haworth CS, Francis S, Miles, J, Clayton N, et al. The effect of rhDNase on exercise performance and gas trapping in adults with cystic fibrosis: a randomised controlled trial. In: 13th International Cystic Fibrosis Congress; 2000 June 4-8; Stockholm. 2000:147. [CFGD REGISTER: BD93]
Frederiksen 2006 {published data only}
-
- Frederiksen B, Koch C, Hoiby N, Pressler T, Hansen A. Effect of aerosolised or rhDNase (Pulmozyme®) on pulmonary infections in CF: an open randomised study. Pediatric Pulmonology 2000;Suppl 20:246. [CFGD REGISTER: BD97a]
-
- Frederiksen B, Pressler T, Hansen A, Koch C, Hoiby N. Effect of aerosolized rhDNase (Pulmozyme) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatrica 2006;65(9):1070-4. [CFGD REGISTER: BD97b] - PubMed
Fuchs 1994 {published data only}
-
- Eisenberg J. Clinical development of rhDNase in the United States [Developpement clinique de la rhDNase aux Etats-Unis]. Archives de Pediatrie 1995;2(7):674-8. [CFGD REGISTER: BD41e] - PubMed
-
- Fuchs HJ, Borowitz D, Christiansen D, Morris E, Nash M, Ramsey B, et al. Aerosolised recombinant human DNase reduces pulmonary exacerbations and improves pulmonary function in patients with cystic fibrosis. In: 36th Annual Conference on Chest Disease; 1993. 1993. [CFGD REGISTER: BD41f]
-
- Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. New England Journal of Medicine 1994;331(10):637-42. [CFGD REGISTER: BD41b] - PubMed
-
- Menzin J, Oster G, Davies L, Drummond MF, Greiner W, Lucioni C et al. A multinational economic evaluation of rhDNase in the treatment of cystic fibrosis. International Journal of Technology Assessment in Healthcare 1996;12(1):52-61. [CFGD REGISTER: BD41g] - PubMed
-
- Oster G, Huse D, Lacey MJ, Regan MM, Fuchs HJ. Effects of recombinant human DNase therapy on healthcare use and costs in patients with cystic fibrosis. Annals of Pharmacotherapy 1995;29(5):459-64. [CFGD REGISTER: BD41d] - PubMed
Laube 1996 {published data only}
-
- Laube BL, Auci RM, Shield DE, Christiansen DH, Lucas MK, Fuchs HJ et al. Effect of rhDNase on airflow obstruction and mucociliary clearance in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 1996;153(2):752-60. [CFGD REGISTER: BD48b] - PubMed
-
- Laube BL, Auci RM, Shields DE, Christiansen D, Fuchs HJ, Rosenstein BJ. A randomized, placebo-controlled trial of the effect of recombinant human DNase (rhDNase) on the deposition homogeneity and mucociliary clearance of radioaerosol in patients with cystic fibrosis. Pediatric Pulmonology 1993;Suppl 9:155-6. [CFGD REGISTER: BD48a]
McCoy 1996 {published data only}
-
- McCoy K, Hamilton S, Johnson CA, for the Pulmozyme study group. Effects of 12-week administration of dornase alfa in patients with advanced cystic fibrosis lung disease. Chest 1996;110(4):889-95. [CFGD REGISTER: BD57b] - PubMed
-
- McCoy K for the Pulmozyme Severe Lung Disease Study Group. rhDNase is well tolerated and effective in cystic fibrosis patients with severe obstructive ling disease. In: 20th European Cystic Fibrosis Conference; 1995 June 18-21; Brussels, Belgium. 1995:L41. [CFGD REGISTER: BD57a]
Minasian 2010 {published data only}
-
- Minasian C, Wallis C, Metcalfe C, Bush A. Comparison of inhaled mannitol, daily rhDNase and a combination of both in children with cystic fibrosis: a randomised trial. Thorax 2010;65(1):51-6. [CFGD REGISTER: BD133b] - PubMed
-
- Minasian CC, Wallis C, Metcalfe C, Bush A. A crossover comparative study of inhaled mannitol, alone and in combination with daily rhDNase, in children with cystic fibrosis. Pediatric Pulmonology 2008;43 Suppl 31:301. [CFGD REGISTER: BD133a]
Paul 2004 {published data only}
-
- Jung A, Shute JK, Chen CIU, Ballmann M, Griese M, Ratjen F, et al. Influence of long-term inhaled rhDNase on free and total IL-8 concentration in BAL fluid in cystic fibrosis. In: 12th European Respiratory Society Annual Congress; 2002 Sep 14-18; Stockholm. 2002:P3285. [CFGD REGISTER: IB21d]
-
- Paul K, Ballmann M, Griese M, Rietschel E, Chen C, Schink T, et al. Effect of rhDNase on endobronchial inflammation in CF patients with mild lung disease: results of multi-center beat study. Pediatric Pulmonology 2002;34 Suppl 24:310-11. [CFGD REGISTER: IB21c]
-
- Paul K, Rietschel E, Ballmann M, Griese M, Worlitzsch D, Shute J, et al. Effect of treatment with dornase alpha on airway inflammation in patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2004;169(6):719-25. [CFGD REGISTER: IB21f] - PubMed
-
- Paul KP, Ballmann M, Dorig G, Griese M, Kleinau I, Ratjen F, et al. Airway inflammation in CF patients with good pulmonary function - baseline data from the multicenter "Beat" study. Pediatric Pulmonology 1998;26 Suppl 17:383-4. [CFGD REGISTER: IB21a]
Quan 2001 {published data only}
-
- Brody A, Molina PL, Klein JS, Campbell JD, Millard SP, Quan J. High-resolution CT is more sensitive to longitudinal decline in lung status in young children with CF than pulmonary function tests. Pediatric Pulmonology 2003;36 Suppl 25:318. [CFGD REGISTER: BD98d]
-
- Grasemann H, Lax H, Treseler JW, Colin AA. Dornase alpha and exhaled NO in cystic fibrosis. Pediatric Pulmonology 2004;38(5):379-85. [CFGD REGISTER: BD150] - PubMed
-
- Konstan MW, Wohl ME, McKenzie S, Sy J, Quan JM, Tiddens HA. A randomized, placebo-controlled trial of two years' treatment with dornase alfa (Pulmozyme®) in cystic fibrosis patients aged 6-10 years with early lung disease. Pediatric Pulmonology 2000;Suppl 20:299-300. [CFGD REGISTER: BD98a]
-
- Quan JM, Tiddens HAWM, Sy JP, McKenzie SG, Montgomery MD, Robinson PJ, et al. A two-year randomized, placebo controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. Journal of Pediatrics 2001;139(6):813-20. [CFGD REGISTER: BD98b] - PubMed
-
- Robinson PJ. Dornase alfa in early cystic fibrosis lung disease. Pediatric Pulmonology 2002;34(3):237-41. [CFGD REGISTER: BD98c] - PubMed
Ramsey 1993 {published data only}
-
- Ramsey BW, Astley SJ, Aitken ML, Burke W, Colin AA, Dorkin HL, et al. Efficacy and safety of short-term administration of aerosolized recombinant human deoxyribonuclease in patients with cystic fibrosis. American Review of Respiratory Disease 1993;148(1):145-51. [CFGD REGISTER: BD37] - PubMed
Ranasinha 1993 {published data only}
-
- Ranasinha C, Assoufi B, Shak S, Christiansen D, Fuchs HJ, Empey D et al. Efficacy and safety of short-term administration of aerosolised recombinant human DNase I in adults with stable stage cystic fibrosis. Lancet 1993;342(8865):199-202. [CFGD REGISTER: BD38b] - PubMed
-
- Ranasinha C, Empey D, Geddes D, Fuchs H, Hodson ME. A phase 2 double-blind placebo controlled study of the pulmonary function and the safety of aerosolised recombinant human DNase in adults with stable stage cystic fibrosis. In: 11th International Cystic Fibrosis Congress; 1992. 1992:LBS5. [CFGD REGISTER: BD38a]
Robinson 2000 {published data only}
-
- Hemming AL, Robinson M, Moriarty C, Bautovich GJ, Bye PTP. Effect of short course of rhDNase on mucociliary clearance in patients with cystic fibrosis. Pediatric Pulmonology 1997;23 Suppl 14:273. [CFGD REGISTER: BD76a] - PubMed
-
- Robinson M, Hemming AL, Moriarty B, Eberl S, Bye PTP. Effect of a short course of rhDNase on cough and mucociliary clearance in patients with cystic fibrosis. Pediatric Pulmonology 2000;30(1):16-24. [CFGD REGISTER: BD76b] - PubMed
Robinson 2005 {published data only}
-
- Robinson T, Leung AN, Northway WH, Blankenberg FG, Chan F, Bloch DA et al. Composite CT/PFT score: an outcome measure which markedly improves sensitivity to change in early cystic fibrosis lung disease. Pediatric Pulmonology 2002;34 Suppl 24:298. [CFGD REGISTER: BD106a]
-
- Robinson TE, Goris ML, Zhu HJ, Chen X, Bhise P, Sheikh F, et al. Dornase alfa reduces air trapping in children with mild cystic fibrosis lung disease: a quantitative analysis. Chest 2005;128(4):2327-35. [CFGD REGISTER: BD106c] - PubMed
-
- Robinson TE, Leung AN, Northway WH, Blankenberg FG, Chan FP, Bloch DA, et al. Composite spirometric-computed tomography outcome measure in early cystic fibrosis lung disease. American Journal of Respiratory and Critical Care Medicine 2003;168(5):588-93. [CFGD REGISTER: BD106b] - PubMed
Shah 1995a {published data only}
-
- Hodson M. Multicenter study of rhDNase in cystic fibrosis with severe pulmonary involvement [Étude multicentrique de la rhDNase dans les mucoviscidoses avec atteinte pulmonaire grave]. Archives de Pediatrie 1995;2(7):679-81. [CFGD REGISTER: BD42c] - PubMed
-
- Shah PI, Bush A, Canny GJ, Colin AA, Fuchs HJ, Geddes DM, et al. Recombinant human DNase I in cystic fibrosis patients with severe pulmonary disease: a short-term, double-blind study followed by six months open-label treatment. European Respiratory Journal 1995;8(6):954-8. [CFGD REGISTER: BD42b] - PubMed
-
- Shah PL, Dewar A, Hodson ME. Scanning electron microscopy of cystic fibrosis sputum. European Respiratory Journal 1995;8(Suppl 19):574S. [CFGD REGISTER: BD38e]
-
- Shah PL, Scott SF, Hodson ME (on behalf of the multicentre study group). Report on a multicentre study using aerosolised recombinant human DNase I in the treatment of cystic fibrosis patients with severe pulmonary disease. Pediatric Pulmonology 1993;16 Suppl 9:157-8. [CFGD REGISTER: BD42a]
-
- Shah PL, Scott SF, Knight RA, Marriott C, Hodson ME. The effects of recombinant human DNase I on sputum DNA content. European Respiratory Journal 1995;8(Suppl 19):574S. [CFGD REGISTER: BD38d]
Suri 2001 {published data only}
-
- Grieve R, Thompson S, Normand C, Suri R, Bush A, Wallis C. A cost-effectiveness analysis of rhDNase in children with cystic fibrosis. International Journal of Technology Assessment in Health Care 2003;19(1):71-9. [CFGD REGISTER: BD96h] - PubMed
-
- Suri R, Marshall LJ, Wallis C, Metcalfe C, Bush A, Shute JK. Effects of recombinant human DNase and hypertonic saline on airway inflammation in children with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2002;166(3):352-5. [CFGD REGISTER: BD96d] - PubMed
-
- Suri R, Metcalfe C, Lees B, Flather M, Normand C, Thompson S, et al. A cross-over comparative study of hypertonic saline alternate day and daily rhDNase in children with Cystic Fibrosis. Thorax 2000;55:A75. [CFGD REGISTER: BD96g]
Wilmott 1996 {published data only}
-
- Wilmott R and the DNase Multicenter Study Group and Genentech staff. A phase II, double blind, multicenter study of the safety and efficacy of aerosolized recombinant human DNase I in hospitalized patients with CF experiencing acute pulmonary exacerbations. Pediatric Pulmonology 1993;16 Suppl 9:154. [CFGD REGISTER: BD49a]
-
- Wilmott RW, Amin RS, Colin AA, De Vault A, Dozor AJ, Eigen H et al. Aerosolized recombinant human DNase in hospitalized cystic fibrosis patients with acute pulmonary exacerbations. American Journal of Respiratory and Critical Care Medicine 1996;153(6 Pt 1):1914-7. [CFGD REGISTER: BD49b] - PubMed
References to studies excluded from this review
Amelina 2019 {published and unpublished data}
-
- Amelina EL, Krasovskiy SA, Abdulganieva DI, Asherova IK, Zil'ber IE, Trishina SV, et al. Efficacy and safety of the biosimilar medicinal product Tigerase® (dornase alfa) in long-term symptomatic treatment of patients with cystic fibrosis: results of a phase III clinical trial. Pulmonologiya 2019;29(6):695-706. [CENTRAL: CN-02130241] [CFGD REGISTER: BD278] [CLINICALTRIALS.GOV: NCT04468100] [EMBASE: 2005791265]
Anderson 2009 {published data only}
-
- Anderson P, Morton J. Evaluation of two different timings of Pulmozyme nebulisation in relation to chest physiotherapy in children with cystic fibrosis. Journal of Cystic Fibrosis 2009;8 Suppl 2:S74. [ABSTRACT NO.: 299] [CFGD REGISTER: BD162]
Bakker 2010 {published data only}
-
- Bakker EM, Volpi S, Salonini E, Mullinger B, Kroneberg P, Hop WCJ, et al. Efficacy of peripheral deposition of inhaled rhDNase in CF patients during a respiratory tract infection. Journal of Cystic Fibrosis 2010;9 Supplement 1:S62. [ABSTRACT NO.: 239] [CFGD REGISTER: BD155a]
-
- Bakker M, Volpi S, Salonini E, Wiel E, Merkus P, Sintnicolaas C, et al. Peripheral versus central deposition of inhaled rhdnase in children with cystic fibrosis. Pediatric Pulmonology 2010;45(S33):306. [ABSTRACT NO.: 244] [CFGD REGISTER: BD155b] 3421199
-
- den Beukel-Bakker M, Volpi S, Salonini E, Wiel-Kooij EC, Sintnicolaas C, Hop WC, et al. Small airways response to dornase alfa improves using controlled inhalation: a randomized controlled trial in cystic fibrosis patients. Journal of Cystic Fibrosis 2011;10 Supplement 1:S19. [ABSTRACT NO.: 75] [CFGD REGISTER: BD155c]
Bilton 2011 {published data only}
-
- Bilton D, Robinson P, Cooper P, Gallagher CG, Kolbe J, Fox H, et al. Inhaled dry powder mannitol in cystic fibrosis: an efficacy and safety study. European Respiratory Journal 2011;38(5):1071-80. [CTG: ] - PubMed
Bishop 2011 {published data only}
-
- Bishop JR, Erskine OJ, Middleton PG. Timing of dornase alpha inhalation does not affect the efficacy of an airway clearance regimen in adults with cystic fibrosis: a randomised crossover trial. Journal of physiotherapy 2011;57(4):223-9. [CFGD REGISTER: BD252a] - PubMed
-
- Middleton PG, Bishop J. Dornase alpha and physiotherapy - which should be first? A randomised double-blind, placebo-controlled trial in CF adults. Pediatric Pulmonology 2001;32 Suppl 22:310. [CFGD REGISTER: BD252b]
Bollert 1999 {published data only}
-
- Bollert FG, McArthur DA, Greening AP, Innes JA on behalf of the Scottish Cystic Fibrosis Group. Targeted introduction of DNase in Scotland. Pediatric Pulmonology 1995;20 Suppl 12:204.
-
- Bollert FG, Paton JY, Marshall TG, Calvert J, Greening AP, Innes JA. Recombinant DNase in cystic fibrosis: a protocol for targeted introduction through n-of-1 trials. Scottish Cystic Fibrosis Group. European Respiratory Journal 1999;13(1):107-13. - PubMed
Cimmino 2005 {published data only}
-
- Cimmino M, Nardone M, Cavaliere M, Plantulli A, Sepe A, Esposito V, et al. Dornase alfa as postoperative therapy in cystic fibrosis sinonasal disease. Archives of Otolaryngology - Head and Neck Surgery 2005;131(12):1097-101. - PubMed
Dab 2000 {published data only}
-
- Dab A, Malfroot D, Baran EM, App M, Coffiner M, Nagy AM. Randomized multicentric double blind study of safety and efficacy of Nacystelyn DPI versus placebo in rhDNase treated cystic fibrosis patients. American Journal of Respiratory and Critical Care Medicine 2000;161(3):A72.
-
- Malfroot A, Dab I, Baran D, App EM, Coffiner M, Nagy AM. Randomised multicentric double blind study of tolerability and efficacy of a DPI Nacystelyn versus placebo in cystic fibrosis patients treated by rhDNase for at least 3 months. In: 13th International Cystic Fibrosis Congress; 2000 June 4-8; Stockholm, Sweden. 2000:146.
-
- Malfroot A, Dab I, Baran D, App EM, Coffiner M, Nagy AM. Randomized multicentric double blind study of tolerability and efficacy of a DPI Nacystelyn versus placebo in cystic fibrosis patients treated by rhDNase for at least 3 months. Pediatric Pulmonology 1999;28 Suppl 19:244.
Elkins 2006 {published data only}
-
- Elkins MR, Bye PTP. Comparison of Pari LC-Star and -Plus nebulisers delivering 2.5mg recombinant human deoxyribonuclease (rhDNase). Journal of Cystic Fibrosis 2006;5 Suppl 1:S42.
EUCTR2006‐002098‐30‐NL {published data only}
-
- EUCTR2006-002098-30-NL. Efficacy of inhaled rhDNase in mechanically ventilated pediatric patients with an atelectasis - rhDNase in ventilated pediatric patients. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006-002098-30-NL (first received 01 June 2006).
EUCTR2007‐000935‐25‐NL {published data only}
-
- EUCTR2007-000935-25-NL. Peripheral targeting of inhaled rhDNase in stable CF patients. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007-000935-25-NL (first received 02 April 2007).
Fitzgerald 2005 {published data only}
-
- Fitzgerald DA, Hilton J, Jepson B, Smith L. A crossover, randomized, controlled trial of dornase alfa before versus after physiotherapy in cystic fibrosis. Pediatrics 2005;116(4):e549-54. [CFGD REGISTER: BD102b] - PubMed
-
- Fitzgerald DA, Hilton J, Smith L, Jepson B. Is dornase alfa (pulmozyme) more effective before or after physiotherapy? A cross-over, randomised placebo controlled trial. Pediatric Pulmonology 2001;32 Suppl 22:309-10. [CFGD REGISTER: BD102a]
Furuya 2001 {published data only}
-
- Furuya ME, Lezana-Fernandez JL, Vargas MH, Hernandez-Sierra JF, Ramirez-Figueroa JL. Efficacy of human recombinant DNase in pediatric patients with cystic fibrosis. Archives of Medical Research 2001;32(1):30-4. - PubMed
Griese 1997 {published data only}
-
- Griese M, App EM, Derouix A, Burkert A, Schams A. Recombinant human DNase (rhDNase) influences phospholipid composition, surface activity, rheology and consecutively clearance indices of cystic fibrosis sputum. Pulmonary Pharmacology and Therapeutics 1997;10(1):21-7. - PubMed
Hagelberg 2008 {published data only}
-
- Hagelberg M, Dooley MJ, Poole SG, Leung D, Bailey M, Finlayson F, et al. Direct dispensing of dornase alpha improves adherence and lung function in cystic fibrosis. Journal of Cystic Fibrosis 2008;7 Suppl 2:S27.
Heijerman 1995 {published data only}
-
- Heijerman HG, Rossem RN, Bakker W. Effect of rhDNase on lung function and quality of life in adult cystic fibrosis patients. The Netherlands Journal of Medicine 1995;46(6):293-7. [CFGD REGISTER: BD43] - PubMed
Hubbard 1992 {published data only}
-
- Hubbard RC, McElvaney NG, Birrer P, Shak S, Robinson WW, Jolley C et al. A preliminary study of aerosolized recombinant human deoxyribonuclease I in the treatment of cystic fibrosis. New England Journal of Medicine 1992;326(12):812-5. - PubMed
Johnson 2006 {published data only}
-
- Johnson JC, Waldrep JC, Dhand R. Aerosol delivery of recombinant human DNase 1. Comparison of a vibrating mesh nebulizer with a jet nebulizer. In: American Thoracic Society International Conference; 2006 May 19-24; California, USA. 2006:A82.
Kelijo 2001 {published data only}
-
- Kelijo DJ, Giroir B, Jialal I. Circulating tumor necrosis factor-alpha and IL-6 levels in patients with cystic fibrosis and mild lung disease: effects of Dnase and alpa-tocopherol therapy. Unpublished article 2001;(Obtained 2014). [CFGD REGISTER: GN87b]
-
- Keljo DJ, Giroir B, Jialal I. Circulating tumor necrosis factor alpha and interleukin-6 levels in cystic fibrosis, effect of vitamin E therapy. Pediatric Pulmonology 2000;30 Suppl 20:326. [CFGD REGISTER: GN87a]
King 1997 {published data only}
-
- King M, Dasgupta B, Tomkiewicz RP, Brown NE, Pulmonary Research Group. Rheology of cystic fibrosis sputum after in vitro treatment with hypertonic saline alone and in combination with recombinant human deoxyribonuclease I. American Journal of Respiratory and Critical Care Medicine 1997;156(1):173-7. - PubMed
Lahiri 2012 {published data only}
-
- Lahiri T, Herrington H, Diehl S, Landrigan G. The effect of intranasal dornase alfa on chronic sinusitis in patients with cystic fibrosis: a pilot study. Pediatric Pulmonology 2012;47 Suppl 35:354.
-
- NCT00416182. Nasally delivered Pulmozyme for sinusitis in cystic fibrosis. clinicaltrials.gov/ct2/show/NCT00416182 (first posted 27 December 2006).
Laube 2005 {published data only}
-
- Laube BL, Geller DE, Lin TC, Dalby RN, Diener-West M, Zeitlin PL. Positive expiratory pressure changes aerosol distribution in patients with cystic fibrosis. Respiratory Care 2005;50(11):1438-44. - PubMed
-
- Laube BL, Lin T, Geller D, Dalby R, Zeitlin P. Positive expiratory pressure alters aerosol distribution in CF. Pediatric Pulmonology 2000;30 Suppl 20:247.
Mainz 2011 {published data only}
-
- Mainz J, Mentzel H, Scheider G, Riethmuller J, Schiller I, Ritschel C, et al. Double-blind, placebo-controlled pilot trial on sinonasal inhalation of dornase alfa in CF. Pediatric Pulmonology 2008;43(Suppl 31):305. [CFGD REGISTER: BD131b] - PubMed
-
- Mainz J, Mentzel HJ, Schneider G, Riethmuller J, Schiller I, Ritschel C, et al. Sinu-nasal inhalation of Dornase alfa in CF. Results of a double-blind placebo-controlled pilot trial. Journal of Cystic Fibrosis 2008;7(Suppl 2):S27. [CFGD REGISTER: BD131a]
-
- Mainz JG, Schiller I, Ritschel, Mentzel HJ, Riethmuller J, Koitschev A, et al. Sinonasal inhalation of dornase alfa in CF: A double-blind placebo-controlled cross-over pilot trial. Auris, Nasus, Larynx 2011;38(2):220-7. [CFGD REGISTER: BD131c] - PubMed
-
- NCT00265434. Nasal inhalation of dornase alfa (pulmozyme) in patients with cystic fibrosis and chronic rhinosinusitis [Nasal inhalation of dornase alfa (pulmozyme) in patients with cystic fibrosis and chronic rhinosinusitis]. clinicaltrials.gov/show/nct00265434 (first received 14 December 2005). [CENTRAL: CN-00757314] [CFGD REGISTER: BD131d]
Mainz 2014 {published data only}
-
- EUCTR2007-001548-36-DE. Nasal inhalation of dornase alfa (pulmozyme) in patients with cystic fibrosis and chronic rhinosinusitis - pulmozyme-nasal-cf [Nasale Inhalation von Pulmozyme bei Patienten mit Mukoviszidose und chronischer Rhinosinusitis mit dem Pari Sinus-Vernebler. - bizentrische, randomisierte, doppel-blinde, placebo-kontrollierte, prospektive klinische Studie]. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007-001548-36-DE (first received 27 July 2009). [CENTRAL: CN-01887259] [CFGD REGISTER: BD154e]
-
- Karadag A, Catal F. Nasal saline as a placebo in chronic rhinosinusitis. Journal of Cystic Fibrosis 2014;13(5):601. [CFGD REGISTER: BD154c] - PubMed
-
- Mainz JG, Schien C, Schiller I, Schadlich K, Koitschev A, Koitschev C, et al. Sinonasal inhalation of dornase alfa administered by vibrating aerosol to cystic fibrosis patients: a double-blind placebo-controlled cross-over trial. Journal of Cystic Fibrosis 2014;13(4):461-70. [CFGD REGISTER: BD154b] - PubMed
-
- Mainz JG, Schiller I, Koitschev A, Koitschev C, Riethmuller J, Wiedemann B, et al. Sinonasal inhalation of dornase alfa reduces rhinosinusitis symptoms in CF. Results of a DBPC-cross-over-study. Journal of Cystic Fibrosis 2010;9 Suppl 1:S23. [ABSTRACT NO.: 88] [CENTRAL: CN-00766447] [CFGD REGISTER: BD154a]
-
- NCT00534079. Nasal inhalation of pulmozyme in patients with cystic fibrosis and chronic rhinosinusitis [Nasal inhalation of dornase alfa (pulmozyme) in patients with cystic fibrosis and chronic rhinosinusitis - a double blind placebo-controlled cross-over, bicenter, prospective clinical study]. clinicaltrials.gov/show/nct00534079 (first received 24 September 2007). [CENTRAL: CN-00757315] [CFGD REGISTER: BD154d]
Majaesic 1996 {published data only}
-
- Majaesic CM, Montgomery M, Jones R, King M. Reduction in sputum viscosity using high frequency chest compressions (HFCC) compared to conventional chest physiotherapy (CCP). Pediatric Pulmonology 1996;22 Suppl 13:308.
Nasr 2001 {published data only}
-
- Nasr SZ, Kuhns LR, Brown RW, Hurwitz ME, Sanders GM, Strouse PJ. Use of computerized tomography and chest-rays in evaluating efficacy of aerosolized recombinant human DNase in cystic fibrosis patients younger than 5 years: A preliminary study. Pediatric Pulmology 2001;31(5):377-82. - PubMed
-
- Nasr SZ, Kuhns LR, Brown RW, Hurwitz RW, Sanders GM, Stouse PJ. Aerolized recombinant human DNase in cystic fibrosis patients younger than 5 years of age. Pediatric Pulmonology 1999;28 Suppl 19:278. - PubMed
NCT00311506 {published data only}
-
- NCT00311506. A study to determine the variability of a 6-minute walk test in cystic fibrosis subjects with advanced lung disease. clinicaltrials.gov/ct2/show/NCT00311506 (first posted 06 April 2006).
NCT00434278 {published data only}
-
- NCT00434278. A trial of pulmozyme withdrawal on exercise tolerance in cystic fibrosis subjects with severe lung disease (TOPIC). clinicaltrials.gov/ct2/show/NCT00434278 (first posted 13 February 2007).
NCT00680316 {published data only}
-
- NCT00680316. A study of Pulmozyme® (dornase alpha) in 3- to 5-year-old patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT00680316 (first posted 20 May 2008).
NCT00843817 {published data only}
-
- NCT00843817. RhDNase and biodistribution of PMN serine proteases in cystic fibrosis sputum (BioDNase). clinicaltrials.gov/ct2/show/NCT00843817 (first posted 13 February 2009).
NCT01025258 {published data only}
-
- NCT01025258. Improving adherence and clinical outcome in cystic fibrosis (CF) patients. clinicaltrials.gov/ct2/show/NCT01025258 (first posted 03 December 2009).
NCT01155752 {published data only}
-
- NCT01155752. Pulmozyme in cystic fibrosis with sinusitis [Z4770s, Use of Recombinant Human DNASE in Cystic Fibrosis Patients With Chronic Sinusitis to Prevent Acute Sinusitis Exacerbations and Improve Symptoms and Outcomes - A Pilot Study]. clinicaltrials.gov/ct2/show/NCT01155752 (first posted 02 July 2010).
NCT01232478 {published data only}
-
- NCT01232478. I change adherence and raise expectations (iCARE). clinicaltrials.gov/ct2/show/NCT01232478 (first posted 02 November 2010).
NCT02301377 {published data only}
-
- NCT02301377. Exploring novel interventions to improve adherence in children with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT02301377 (first posted 25 November 2014).
NCT02682290 {published data only}
-
- NCT02682290. Assessment of rheological parameters of human sputum (RHEOMUCO). clinicaltrials.gov/ct2/show/NCT02682290 (first posted 15 February 2016).
NCT02722122 {published data only}
-
- NCT02722122. Study to evaluate the safety,tolerability, pharmacokinetics and exploratory efficacy parameters of AIR DNase™ in patients with cystic fibrosis previously treated with Pulmozyme®. clinicaltrials.gov/ct2/show/NCT02722122 (first posted 29 March 2016).
Potter 2008 {published data only}
-
- Potter RW, Hurren TJ, Nickerson C, Hatley RH. Comparison of the delivery characteristics of Dornase alfa from the I-NEB AAD system and the sidestream jet nebulizer. Pediatric Pulmonology 2008;43 Suppl 31:361.
QUEST {published data only}
-
- Qualitative understanding of experiences with the SIMPLIFY Trial (QUEST). clinicaltrials.gov/ct2/show/NCT04320381 first posted 25 March 2020.
Riethmueller 2006 {published data only}
-
- Riethmueller J, Borth-Bruhns T, Kumpf M, Vonthein R, Wiskirchen J, Stern M, et al. Recombinant human deoxyribonuclease shortens ventilation time in young, mechanically ventilated children. Pediatric Pulmonology 2006;41(1):61-6. - PubMed
Robinson 2002 {published data only}
-
- Robinson T, Goris ML, Bhise P, Sathi A, Zhu JH, Moss RB. Quantitative HRCT air trapping analysis in CF subjects with mild lung disease during a pulmozyme intervention study. Pediatric Pulmonology 2002;34 Suppl 24:298. [CFGD REGISTER: MU94]
Sawicki 2014 {published data only}
-
- Konstan MW, Chou W, Trzaskoma B, Xu Y. A study to evaluate the comparable efficacy and safety of Pulmozyme® delivered by the Erapid™ nebulizer system. Pediatric Pulmonology 2013;48 Suppl 36:452. [ABSTRACT NO.: 668] [CENTRAL: 999883] [CFGD REGISTER: BD219c]
-
- Sawicki GS, Chou W, Raimundo K, Trzaskoma B, Konstan MW. Randomized trial of efficacy and safety of dornase alfa delivered by eRapid nebulizer in cystic fibrosis patients. Journal of Cystic Fibrosis 2015;14(6):777-83. [CFGD REGISTER: BD219d] - PubMed
-
- Sawicki GS, Konstan M, Chou W, Trzaskoma B, Raimundo K. Impact of dornase alfa delivered by the eRapid™ vs. Pari LC® plus jet nebulizer system on cystic fibrosis-related quality of life - results from the IMPART Study. Pediatric Pulmonology 2014;49 Suppl 38:445-6. [ABSTRACT NO.: 621] [CENTRAL: 1012526] [CFGD REGISTER: BD219a]
-
- Sawicki GS, Konston M, Chou W, Trzaskoma B, Raimundo K. Treatment preferences and satisfaction with dornase alfa delivered by the eRapid™ vs. Pari LC® plus jet nebulizer system to patients with cystic fibrosis - results from the IMPART Study. Pediatric Pulmonology 2014;49 Suppl 38:444. [ABSTRACT NO.: 617] [CENTRAL: 1012527] [CFGD REGISTER: BD219b]
Shah 1995b {published data only}
-
- Shah PL, Scott SF, Geddes DM, Hodson ME. Two years of experience with recombinant human DNase I in the treatment of pulmonary disease in cystic fibrosis. Respiratory Medicine 1995;89(7):499-502. [CFGD REGISTER: BD132] - PubMed
Shah 1995c {published data only}
Shah 1997 {published data only}
-
- Shah PL, Scott SF, Geddes DM, Conway S, Carr S, Wallis C, et al. An evaluation of two aerosol delivery systems for rhDNase [abstract]. Israel Journal of Medical Sciences 1996;32(Suppl):S222. [CFGD REGISTER: DT11a] - PubMed
-
- Shah PL, Scott SF, Geddes DM, Conway S, Watson A, Nazir T, et al. An evaluation of two aerosol delivery systems for rhDNase. European Respiratory Journal 1997;10(6):1261-6. [CFGD REGISTER: DT11b] - PubMed
Tarrant 2019 {published data only}
-
- Tarrant B, Snell G, Ivulich S, Button B, Thompson B, Holland A. Dornase alfa during lower respiratory tract infection post lung transplantation. Respirology 2018;23(Supplement 1):50. [CENTRAL: CN-01607181] [CFGD REGISTER: BD279a] [EMBASE: 622091701] - PubMed
-
- Tarrant BJ, Snell G, Ivulich S, Button B, Thompson B, Holland A. Dornase alfa during lower respiratory tract infection post-lung transplantation: a randomized controlled trial. Transplant International 2019;32(6):603-13. [CENTRAL: CN-02078441] [CFGD REGISTER: BD279b] [DOI: 10.1111/tri.13400] [PMID: ] - DOI - PubMed
ten Berge 2003 {published data only}
-
- ten Berge M, Wiel E, Tiddens HA, Merkus PJ, Hop WC, Jongste JC. DNase in stable cystic fibrosis infants: a pilot study. Journal of Cystic Fibrosis 2003;2(4):183-8. - PubMed
van der Giessen 2007a {published data only}
-
- Giessen L. Does the timing of inhaled dornase alfa matter? Journal of Cystic Fibrosis 2009;8(Suppl 1):S6-9. [CFGD REGISTER: BD116c // BD126c] - PubMed
-
- Giessen LJ, Jongste JC, Gosselink R, Hop WC, Tiddens HA. RhDNase before airway clearance therapy improves airway patency in children with CF. Pediatric Pulmonology 2007;42(7):624-30. [CFGD REGISTER: BD116b] - PubMed
-
- Giessen LJ, Gosselink R, Jongste JC, Hop WC, Tiddens HA. Timing of nebulisation of rhDNase and airway clearance techniques (ACT) in children with Cystic Fibrosis. Journal of Cystic Fibrosis 2005;4 Suppl:S97. [CFGD REGISTER: BD116b]
van der Giessen 2007b {published data only}
-
- Giessen L. Does the timing of inhaled dornase alfa matter? Journal of Cystic Fibrosis 2009;8(Suppl 1):S6-9. [CFGD REGISTER: BD116c // BD126c] - PubMed
-
- Giessen LJ, Bakker M, Hop WC, Tiddens HA. Nocturnal saturation in children with cystic fibrosis. In: European Respiratory Society Annual Congress; 2006 sep 2-6; Munich, Germany. 2006. [ABSTRACT NO.: P4106] [CFGD REGISTER: BD126d]
-
- Giessen LJ, Gosselink R, Hop W, Tiddens H. RhDNase before or after going to sleep in children with cystic fibrosis? Journal of Cystic Fibrosis 2007;6 Suppl 1:S68 Poster 274. [CFGD REGISTER: BD126a]
-
- Giessen LJ, Gosselink R, Hop WC, Tiddens HA. Recombinant human DNase nebulisation in children with cystic fibrosis: before bedtime or after waking up? European Respiratory Journal 2007;30(4):763-8. [CFGD REGISTER: BD126b] - PubMed
Weck 1999 {published data only}
-
- Weck MB, Retsch-Bogart GZ, Scott CS. Efficacy of DNase in individual children using the N-of-1 study design. Pediatric Pulmonology 1999;28 Suppl 19:285.
Wilson 2007 {published data only}
-
- Wilson CJ, Robbins LJ, Murphy JM, Chang AB. Is a longer time interval between recombinant human deoxyribonuclease (dornase alfa) and chest physiotherapy better? A multi-center, randomized crossover trial. Pediatric Pulmonology 2007;42(12):1110-6. - PubMed
References to ongoing studies
SIMPLIFY {published data only}
-
- Impact of discontinuing chronic therapies in people with cystic fibrosis on highly effective CFTR modulator therapy (SIMPLIFY). clinicaltrials.gov/ct2/show/NCT04378153 first posted 07 May 2020.
-
- Mayer-Hamblett N, Nichols DP, Odem-Davis K, Riekert KA, Sawicki GS, Donaldson SH, et al. Evaluating the impact of stopping chronic therapies after modulator drug therapy in cystic fibrosis: the SIMPLIFY study design. Annals of the American Thoracic Society 2021 Jan 19 [Epub ahead of print]. [DOI: 10.1513/AnnalsATS.202010-1336SD] [PMID: ] - DOI - PMC - PubMed
Additional references
Cho E 2015 [pers comm]
-
- Cho E. Cost of medications [personal communication]. Email to: C Yang 15 April 2015.
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. - PubMed
Flume 2007
-
- Flume PA, O'Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Willey-Courand DB, et al. Cystic Fibrosis Pulmonary Guidelines: Chronic Medications for Maintenance of Lung Health. American Journal of Respiratory and Critical Care Medicine 2007;176(10):957-69. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Altman DG on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Jacques 2008
-
- Jaques A, Daviskas E, Turton JA, McKay K, Cooper P, Stirling RG, et al. Inhaled mannitol improves lung function in cystic fibrosis. Chest 2008;133(6):1388-96. - PubMed
Jones 2009
-
- Jones AP, Riley RD, Williamson PR, Whitehead A. Meta-analysis of individual patient data versus aggregate data from longitudinal clinical trials. Clinical Trials 2009;6(1):16-27. - PubMed
Lieberman 1968
-
- Lierberman J. Dornase aerosol effect on sputum viscosity in cases of cystic fibrosis. Journal of the American Medical Association 1968;205:312-3. - PubMed
Menzin 1996
-
- Menzin J, Oster G, Davies L, Drummond MF, Greiner W, Lucioni C, et al. A multi national economic evaluation of rhDNase in the treatment of cystic fibrosis. International Journal of Technology Assessment in Healthcare 1996;12(1):52-61. - PubMed
NICE 2012
-
- National Institute for Health and Clinical Excellence. Mannitol dry powder for inhalation for treating cystic fibrosis. www.nice.org.uk/guidance/ta266/documents/cystic-fibrosis-mannitol-final-... (accessed 30 October 2012):1-62.
Oster 1995
-
- Oster G, Huse D, Lacey MJ, Regan MM, Fuchs HJ. Effects of recombinant human DNase therapy on healthcare use and costs in patients with cystic fibrosis. Annals of Pharmacotherapy 1995;29(5):459-64. - PubMed
Sawicki 2009
Shteinberg 2020
Sly 2009
-
- Sly P, Brennan S, Gangell C, Klerk N, Murray C, Mott L, et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. American Journal of Respiratory and Critical Care Medicine 2009;180(2):146-52. - PubMed
von der Schulenburg 1995
-
- Schulenburg JM, Greiner W, Hardt H. Socioeconomic evaluation of the influence of rhDNase on the costs of treating respiratory tract infections in patients with cystic fibrosis [Soziookonomische Evaluation des Einflusses von rhDNase auf die Kosten der Behandlung von Infektionen der Atemwege bei Patienten mit zystischer Fibrose]. Medizinische Klinik 1995;90(4):220-4. - PubMed
References to other published versions of this review
Jones 2003
Jones 2010
Kearney 1998
Yang 2016
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

