Three-dimensional microscopy and image fusion reconstruction analysis of the thyroid gland during morphogenesis
- PMID: 33735512
- PMCID: PMC8091578
- DOI: 10.1002/2211-5463.13150
Three-dimensional microscopy and image fusion reconstruction analysis of the thyroid gland during morphogenesis
Abstract
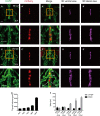
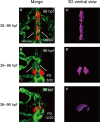
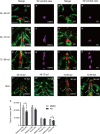
Thyroid dysgenesis (TD) is a major cause of primary congenital hypothyroidism; however, the molecular mechanism underlying this process is unclear. Current knowledge regarding the morphogenesis of the thyroid gland and vascular anomalies affecting thyroid development is limited. To monitor the early stages of thyroid gland development, we generated double transgenic zebrafish embryos Tg(tg:mCherry/flk1:EGFP). We described the volume of the thyroid from 2 days postfertilization (dpf) to 5 dpf using 3D reconstruction images. We treated zebrafish embryos with the fibroblast growth factor (FGF) inhibitor PD166866 to better understand the impact of vascular defects on thyroid development and the effects of drug administration at specific time periods on different stages of thyroid development. The 3D reconstruction data revealed that the thyroid glands underwent significant transformation at critical time points. PD166866 treatment from 48 to 72 hours postfertilization (hpf) and from 72 to 96 hpf did not cause obvious reductions in thyroid volume but did result in observable abnormalities in thyroid morphology. The treatment also affected thyroid volume from 36 to 48 hpf, thus indicating that there are time-point-specific effects of drug administration during thyroid development. Three-dimensional image reconstruction provides a comprehensive picture of thyroid anatomy and can be used to complement anatomical fluorescence information. The effects of an FGF pathway inhibitor on thyroid development were determined to be time-point-dependent.
Keywords: 3D reconstruction; Zebrafish; embryonic development; endoderm; fibroblast growth factors; thyroid gland.
© 2021 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Enhanced Canonical Wnt Signaling During Early Zebrafish Development Perturbs the Interaction of Cardiac Mesoderm and Pharyngeal Endoderm and Causes Thyroid Specification Defects.Thyroid. 2021 Mar;31(3):420-438. doi: 10.1089/thy.2019.0828. Epub 2020 Sep 16. Thyroid. 2021. PMID: 32777984
-
Small-Molecule Screening in Zebrafish Embryos Identifies Signaling Pathways Regulating Early Thyroid Development.Thyroid. 2019 Nov;29(11):1683-1703. doi: 10.1089/thy.2019.0122. Epub 2019 Oct 8. Thyroid. 2019. PMID: 31507237
-
Transgenic zebrafish illuminate the dynamics of thyroid morphogenesis and its relationship to cardiovascular development.Dev Biol. 2012 Dec 15;372(2):203-16. doi: 10.1016/j.ydbio.2012.09.011. Epub 2012 Sep 26. Dev Biol. 2012. PMID: 23022354
-
Morphogenesis of the thyroid gland.Mol Cell Endocrinol. 2010 Jul 8;323(1):35-54. doi: 10.1016/j.mce.2009.12.008. Epub 2009 Dec 21. Mol Cell Endocrinol. 2010. PMID: 20026174 Review.
-
Morphogenetics of early thyroid development.J Mol Endocrinol. 2011 Feb;46(1):R33-42. doi: 10.1677/jme-10-0084. J Mol Endocrinol. 2011. PMID: 21322126 Review.
Cited by
-
Disruption of the foxe1 gene in zebrafish reveals conserved functions in development of the craniofacial skeleton and the thyroid.Front Cell Dev Biol. 2023 Mar 13;11:1143844. doi: 10.3389/fcell.2023.1143844. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36994096 Free PMC article.
References
-
- Mio C, Grani G, Durante C and Damante G (2020) Molecular defects in thyroid dysgenesis. Clin Genet 97, 222–231. - PubMed
-
- Mass Screening Committee; Japanese Society for Pediatric Endocrinology , Nagasaki K, Minamitani K, Anzo M, Adachi M, Ishii T, Onigata K, Kusuda S, Harada S, Horikawa R, Minagawa M et al. (2015) Guidelines for mass screening of congenital hypothyroidism (2014 revision). Clin Pediatr Endocrinol 24, 107–133. - PMC - PubMed
-
- Deladoëy J, Bélanger N and Van Vliet G (2007) Random variability in congenital hypothyroidism from thyroid dysgenesis over 16 years in Québec. J Clin Endocrinol Metab 92, 3158–3161. - PubMed
-
- Yamaguchi T, Nakamura A, Nakayama K, Hishimura N, Morikawa S, Ishizu K and Tajima T (2020) Targeted next‐generation sequencing for congenital hypothyroidism with positive neonatal TSH screening. J Clin Endocrinol Metab 105, e2825–e2833. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

