Molecular and genetic biomarkers implemented from next-generation sequencing provide treatment insights in clinical practice for Waldenström macroglobulinemia
- PMID: 33735664
- PMCID: PMC7985670
- DOI: 10.1016/j.neo.2021.02.002
Molecular and genetic biomarkers implemented from next-generation sequencing provide treatment insights in clinical practice for Waldenström macroglobulinemia
Abstract
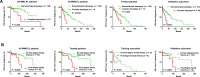
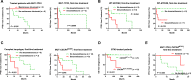
Waldenström macroglobulinemia (WM) is a distinct type of indolent lymphoplasmacytic lymphoma (LPL) with a high frequency of MYD88L265P mutation. Treatment for WM/LPL is highly variable in clinic and ibrutinib (a Bruton tyrosine kinase inhibitor, BTKi) has become a new treatment option for WM. To investigate the clinical impact of genetic alterations in WM, we assembled a large cohort of 219 WMs and 12 LPLs dividing into two subcohorts: a training cohort, patients sequenced by a same targeted 29-gene next-generation sequencing (NGS) panel, and a validation cohort, patients sequenced by allele specific-PCR or other targeted NGS panels. In both training and validation subcohorts, MYD88L265P and TP53 mutations showed favorable and adverse prognostic effects, respectively. CXCR4 nonsense/missense mutations (CXCR4NS/MS), cytogenetic complex karyotypes, and a family history of lymphoma/leukemia in first-degree relatives were associated with significantly worse clinical outcomes only or more in the validation subcohort. We further investigated the efficacy of various treatments and interaction with genetic factors in the entire cohort. Upfront dexamethasone usage was associated with poorer clinical outcomes in patients who received non-proteasome-containing chemotherapy as first-line treatment independent of genetic factors. Maintenance rituximab was associated with better survival. Ibrutinib/BTKi showed potential benefit in relapsed/refractory patients and patients without CXCR4NS/MS including those with TP53 mutations. In conclusion, genetic testing for MYD88L265P, TP53, and CXCR4 mutations and cytogenetic analysis provide important information for prognosis prediction and therapy selection. The findings in these study are valuable for improving treatment decisions on therapies available for WM/LPL patients with integration of NGS in clinic.
Keywords: CXCR4; Cytogenetic karyotype; Ibrutinib; MYD88; TP53; Waldenström macroglobulinemia.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Owen R.G., Treon S.P., Al-Katib A., Fonseca R., Greipp P.R., McMaster M.L., Morra E., Pangalis G.A., San Miguel J.F., Branagan A.R. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30(2):110–115. - PubMed
-
- Ansell S.M., Kyle R.A., Reeder C.B., Fonseca R., Mikhael J.R., Morice W.G., Bergsagel P.L., Buadi F.K., Colgan J.P., Dingli D. Diagnosis and management of Waldenström macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines. Mayo Clin Proc. 2010;85(9):824–833. - PMC - PubMed
-
- Kapoor P., Ansell S.M., Fonseca R., Chanan-Khan A., Kyle R.A., Kumar S.K., Mikhael J.R., Witzig T.E., Mauermann M., Dispenzieri A. Diagnosis and Management of Waldenström Macroglobulinemia: Mayo Stratification of Macroglobulinemia and Risk-Adapted Therapy (mSMART) Guidelines 2016. JAMA Oncol. 2017;3(9):1257–1265. - PMC - PubMed
-
- Dimopoulos M.A., Trotman J., Tedeschi A., Matous J.V., Macdonald D., Tam C., Tournilhac O., Ma S., Oriol A., Heffner L.T. Ibrutinib for patients with rituximab-refractory Waldenstrom's macroglobulinaemia (iNNOVATE): an open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. 2017;18(2):241–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

