Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia
- PMID: 33735712
- PMCID: PMC7951885
- DOI: 10.1016/j.intimp.2021.107522
Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia
Abstract
Background: We examined the safety and efficacy of a treatment protocol containing Favipiravir for the treatment of SARS-CoV-2.
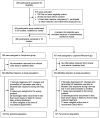
Methods: We did a multicenter randomized open-labeled clinical trial on moderate to severe cases infections of SARS-CoV-2. Patients with typical ground glass appearance on chest computerized tomography scan (CT scan) and oxygen saturation (SpO2) of less than 93% were enrolled. They were randomly allocated into Favipiravir (1.6 gr loading, 1.8 gr daily) and Lopinavir/Ritonavir (800/200 mg daily) treatment regimens in addition to standard care. In-hospital mortality, ICU admission, intubation, time to clinical recovery, changes in daily SpO2 after 5 min discontinuation of supplemental oxygen, and length of hospital stay were quantified and compared in the two groups.
Results: 380 patients were randomly allocated into Favipiravir (193) and Lopinavir/Ritonavir (187) groups in 13 centers. The number of deaths, intubations, and ICU admissions were not significantly different (26, 27, 31 and 21, 17, 25 respectively). Mean hospital stay was also not different (7.9 days [SD = 6] in the Favipiravir and 8.1 [SD = 6.5] days in Lopinavir/Ritonavir groups) (p = 0.61). Time to clinical recovery in the Favipiravir group was similar to Lopinavir/Ritonavir group (HR = 0.94, 95% CI 0.75 - 1.17) and likewise the changes in the daily SpO2 after discontinuation of supplemental oxygen (p = 0.46) CONCLUSION: Adding Favipiravir to the treatment protocol did not reduce the number of ICU admissions or intubations or In-hospital mortality compared to Lopinavir/Ritonavir regimen. It also did not shorten time to clinical recovery and length of hospital stay.
Keywords: Clinical trial; Covid19; Favipiravir; Hydroxychloroquine; Lopinavir; Ritonavir; SARS-CoV-2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Comment in
-
Comment on: Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia.Int Immunopharmacol. 2022 Jan;102:107693. doi: 10.1016/j.intimp.2021.107693. Epub 2021 May 28. Int Immunopharmacol. 2022. PMID: 34217670 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous