The lung immuno-oncology prognostic score (LIPS-3): a prognostic classification of patients receiving first-line pembrolizumab for PD-L1 ≥ 50% advanced non-small-cell lung cancer
- PMID: 33735802
- PMCID: PMC7988288
- DOI: 10.1016/j.esmoop.2021.100078
The lung immuno-oncology prognostic score (LIPS-3): a prognostic classification of patients receiving first-line pembrolizumab for PD-L1 ≥ 50% advanced non-small-cell lung cancer
Erratum in
-
Corrigendum to 'The lung immuno-oncology prognostic score (LIPS-3): a prognostic classification of patients receiving first-line pembrolizumab for PD-L1 ≥ 50% advanced non-small-cell lung cancer': [ESMO Open Volume 6, Issue 2, April 2021, 100078].ESMO Open. 2021 Jun;6(3):100137. doi: 10.1016/j.esmoop.2021.100137. Epub 2021 Jun 3. ESMO Open. 2021. PMID: 34144775 Free PMC article. No abstract available.
Abstract
Background: To stratify the prognosis of patients with programmed cell death-ligand 1 (PD-L1) ≥ 50% advanced non-small-cell lung cancer (aNSCLC) treated with first-line immunotherapy.
Methods: Baseline clinical prognostic factors, the neutrophil-to-lymphocyte ratio (NLR), PD-L1 tumour cell expression level, lactate dehydrogenase (LDH) and their combination were investigated by a retrospective analysis of 784 patients divided between statistically powered training (n = 201) and validation (n = 583) cohorts. Cut-offs were explored by receiver operating characteristic (ROC) curves and a risk model built with validated independent factors by multivariate analysis.
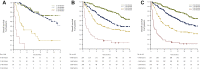
Results: NLR < 4 was a significant prognostic factor in both cohorts (P < 0.001). It represented 53% of patients in the validation cohort, with 1-year overall survival (OS) of 76.6% versus 44.8% with NLR > 4, in the validation series. The addition of PD-L1 ≥ 80% (21% of patients) or LDH < 252 U/l (25%) to NLR < 4 did not result in better 1-year OS (of 72.6% and 74.1%, respectively, in the validation cohort). Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 2 [P < 0.001, hazard ratio (HR) 2.04], pretreatment steroids (P < 0.001, HR 1.67) and NLR < 4 (P < 0.001, HR 2.29) resulted in independent prognostic factors. A risk model with these three factors, namely, the lung immuno-oncology prognostic score (LIPS)-3, accurately stratified three OS risk-validated categories of patients: favourable (0 risk factors, 40%, 1-year OS of 78.2% in the whole series), intermediate (1 or 2 risk factors, 54%, 1-year OS 53.8%) and poor (>2 risk factors, 5%, 1-year OS 10.7%) prognosis.
Conclusions: We advocate the use of LIPS-3 as an easy-to-assess and inexpensive adjuvant prognostic tool for patients with PD-L1 ≥ 50% aNSCLC.
Keywords: LDH; PD-L1; immune-checkpoint inhibitor; immunotherapy; neutrophil-to-lymphocyte ratio; non-small-cell lung cancer; performance status; prognostic; steroids.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure AC received speaker fees and grant consultancies from AstraZeneca, MSD, BMS, Roche, Novartis and Astellas. EB received speaker and travel fees from MSD, Astra-Zeneca, Pfizer, Helsinn, Eli-Lilly, BMS, Novartis and Roche; received grant consultancies from Roche and Pfizer. MT received speaker fees and grant consultancies from AstraZeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Otsuka, Takeda and Pierre Fabre. AM received speaker fees from Astra, Roche, BMS, MSD, Boehringer, Pfizer and Takeda. FM received grant consultancies from MSD and Takeda. RG received speaker fees and grant consultancies from AstraZeneca and Roche. AF received grant consultancies from Roche, Pfizer, Astellas and BMS. AA received grant consultancies from Takeda, MSD, BMJ, AstraZeneca, Roche and Pfizer. RC received speaker fees from BMS, MSD, Takeda, Pfizer, Roche and AstraZeneca. CG received speaker fees/grant consultancies from Astra Zeneca, BMS and Boehringer-Ingelheim. GLB personal fees from Janssen Cilag, Boehringer Ingelheim, AstraZeneca and Roche, outside the submitted work. All other authors have declared no conflicts of interest. Data sharing The datasets used during this study are available from the corresponding author upon reasonable request.
Figures



References
-
- Banna G.L., Passiglia F., Colonese F. Immune-checkpoint inhibitors in non-small cell lung cancer: a tool to improve patients' selection. Crit Rev Oncol Hematol. 2018;129:27–39. - PubMed
-
- Reck M., Rodriguez-Abreu D., Robinson A.G. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–546. - PubMed
-
- Hellmann M.D., Paz-Ares L., Bernabe Caro R. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. - PubMed
-
- Paz-Ares L., Ciuleanu T.E., Cobo M. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

