In vitro effects-based method and water quality screening model for use in pre- and post-distribution treated waters
- PMID: 33736315
- PMCID: PMC8085790
- DOI: 10.1016/j.scitotenv.2020.144750
In vitro effects-based method and water quality screening model for use in pre- and post-distribution treated waters
Abstract
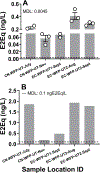
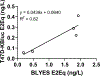
Recent urban public water supply contamination events emphasize the importance of screening treated drinking water quality after distribution. In vitro bioassays, when run concurrently with analytical chemistry methods, are effective tools to evaluating the efficacy of water treatment processes and water quality. We tested 49 water samples representing the Chicago Department of Water Management service areas for estrogen, (anti)androgen, glucocorticoid receptor-activating contaminants and cytotoxicity. We present a tiered screening approach suitable to samples with anticipated low-level activity and initially tested all extracts for statistically identifiable endocrine activity; performing a secondary dilution-response analysis to determine sample EC50 and biological equivalency values (BioEq). Estrogenic activity was detected in untreated Lake Michigan intake water samples using mammalian (5/49; median: 0.21 ng E2Eq/L) and yeast cell (5/49; 1.78 ng E2Eq/L) bioassays. A highly sensitive (anti)androgenic activity bioassay was applied for the first time to water quality screening and androgenic activity was detected in untreated intake and treated pre-distribution samples (4/49; 0.93 ng DHTEq/L). No activity was identified above method detection limits in the yeast androgenic, mammalian anti-androgenic, and both glucocorticoid bioassays. Known estrogen receptor agonists were detected using HPLC/MS-MS (estrone: 0.72-1.4 ng/L; 17α-estradiol: 1.3-1.5 ng/L; 17β-estradiol: 1.4 ng/L; equol: 8.8 ng/L), however occurrence did not correlate with estrogenic bioassay results. Many studies have applied bioassays to water quality monitoring using only relatively small samples sets often collected from surface and/or wastewater effluent. However, to realistically adapt these tools to treated water quality monitoring, water quality managers must have the capacity to screen potentially hundreds of samples in short timeframes. Therefore, we provided a tiered screening model that increased sample screening speed, without sacrificing statistical stringency, and detected estrogenic and androgenic activity only in pre-distribution Chicago area samples.
Keywords: Androgen; Effects-based method; Estrogen; Tapwater.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
De Facto Water Reuse: Bioassay suite approach delivers depth and breadth in endocrine active compound detection.Sci Total Environ. 2020 Jan 10;699:134297. doi: 10.1016/j.scitotenv.2019.134297. Epub 2019 Sep 4. Sci Total Environ. 2020. PMID: 31683213 Free PMC article.
-
Analysis of the sensitivity of in vitro bioassays for androgenic, progestagenic, glucocorticoid, thyroid and estrogenic activity: Suitability for drinking and environmental waters.Environ Int. 2017 Feb;99:120-130. doi: 10.1016/j.envint.2016.12.014. Epub 2016 Dec 22. Environ Int. 2017. PMID: 28017361 Review.
-
Endocrine disrupting activities in sewage effluent and river water determined by chemical analysis and in vitro assay in the context of granular activated carbon upgrade.Chemosphere. 2011 Sep;84(10):1512-20. doi: 10.1016/j.chemosphere.2011.04.032. Epub 2011 May 5. Chemosphere. 2011. PMID: 21546050
-
Exploring potential contributors to endocrine disrupting activities in Taiwan's surface waters using yeast assays and chemical analysis.Chemosphere. 2015 Nov;138:814-20. doi: 10.1016/j.chemosphere.2015.08.016. Epub 2015 Aug 24. Chemosphere. 2015. PMID: 26295540
-
In vitro bioassays to screen for endocrine active pharmaceuticals in surface and waste waters.J Pharm Biomed Anal. 2015 Mar 15;106:107-15. doi: 10.1016/j.jpba.2014.11.018. Epub 2014 Nov 20. J Pharm Biomed Anal. 2015. PMID: 25555519 Review.
Cited by
-
Juxtaposition of intensive agriculture, vulnerable aquifers, and mixed chemical/microbial exposures in private-well tapwater in northeast Iowa.Sci Total Environ. 2023 Apr 10;868:161672. doi: 10.1016/j.scitotenv.2023.161672. Epub 2023 Jan 16. Sci Total Environ. 2023. PMID: 36657670 Free PMC article.
-
Recent Trends in Multiclass Analysis of Emerging Endocrine Disrupting Contaminants (EDCs) in Drinking Water.Molecules. 2022 Dec 13;27(24):8835. doi: 10.3390/molecules27248835. Molecules. 2022. PMID: 36557967 Free PMC article. Review.
-
Exposures and potential health implications of contaminant mixtures in linked source water, finished drinking water, and tapwater from public-supply drinking water systems in Minneapolis/St. Paul area, USA.Environ Sci (Camb). 2023 Jul 1;9(7):1813-1828. doi: 10.1039/d3ew00066d. Epub 2023 May 11. Environ Sci (Camb). 2023. PMID: 40546683 Free PMC article.
-
Water, Water Everywhere, but Every Drop Unique: Challenges in the Science to Understand the Role of Contaminants of Emerging Concern in the Management of Drinking Water Supplies.Geohealth. 2023 Dec 28;7(12):e2022GH000716. doi: 10.1029/2022GH000716. eCollection 2023 Dec. Geohealth. 2023. PMID: 38155731 Free PMC article. Review.
-
Bottled water contaminant exposures and potential human effects.Environ Int. 2023 Jan;171:107701. doi: 10.1016/j.envint.2022.107701. Epub 2022 Dec 15. Environ Int. 2023. PMID: 36542998 Free PMC article.
References
-
- Blackwell BR, Ankley GT, Bradley PM, Houck KA, Makarov SS, Medvedev AV, Swintek J, & Villeneuve DL (2019). Potential Toxicity of Complex Mixtures in Surface Waters from a Nationwide Survey of United States Streams: Identifying in Vitro Bioactivities and Causative Chemicals. Environmental Science & Technology, 53(2), 973–983. doi: 10.1021/acs.est.8b05304 - DOI - PMC - PubMed
-
- Blackwell BR, Ankley GT, Corsi SR, DeCicco LA, Houck KA, Judson RS, Li S, Martin MT, Murphy E, Schroeder AL, Smith ER, Swintek J, & Villeneuve DL (2017). An “EAR” on Environmental Surveillance and Monitoring: A Case Study on the Use of Exposure–Activity Ratios (EARs) to Prioritize Sites, Chemicals, and Bioactivities of Concern in Great Lakes Waters. Environmental Science & Technology, 51(15), 8713–8724. doi: 10.1021/acs.est.7b01613 - DOI - PMC - PubMed
-
- Box GEP, Hunter WG, & S HJ (1978). Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building. New York, NY: Wiley & Sons Inc.
-
- Brack W, Altenburger R, Schüürmann G, Krauss M, López Herráez D, van Gils J, Slobodnik J, Munthe J, Gawlik BM, van Wezel A, Schriks M, Hollender J, Tollefsen KE, Mekenyan O, Dimitrov S, Bunke D, Cousins I, Posthuma L, van den Brink PJ, López de Alda M, Barceló D, Faust M, Kortenkamp A, Scrimshaw M, Ignatova S, Engelen G, Massmann G, Lemkine G, Teodorovic I, Walz K-H, Dulio V, Jonker MTO, Jäger F, Chipman K, Falciani F, Liska I, Rooke D, Zhang X, Hollert H, Vrana B, Hilscherova K, Kramer K, Neumann S, Hammerbacher R, Backhaus T, Mack J, Segner H, Escher B, & de Aragão Umbuzeiro G. (2015). The SOLUTIONS project: Challenges and responses for present and future emerging pollutants in land and water resources management. Science of The Total Environment, 503–504, 22–31. doi: 10.1016/j.scitotenv.2014.05.143 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

