Phytochelatins as a Dynamic System for Cd(II) Buffering from the Micro- to Femtomolar Range
- PMID: 33736430
- PMCID: PMC8041291
- DOI: 10.1021/acs.inorgchem.0c03639
Phytochelatins as a Dynamic System for Cd(II) Buffering from the Micro- to Femtomolar Range
Abstract
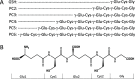
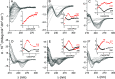
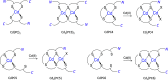
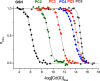
Phytochelatins (PCs) are short Cys-rich peptides with repeating γ-Glu-Cys motifs found in plants, algae, certain fungi, and worms. Their biosynthesis has been found to be induced by heavy metals-both biogenic and toxic. Among all metal inducers, Cd(II) has been the most explored from a biological and chemical point of view. Although Cd(II)-induced PC biosynthesis has been widely examined, still little is known about the structure of Cd(II) complexes and their thermodynamic stability. Here, we systematically investigated glutathione (GSH) and PC2-PC6 systems, with regard to their complex stoichiometries and spectroscopic and thermodynamic properties. We paid special attention to the determination of stability constants using several complementary techniques. All peptides form CdL complexes, but CdL2 was found for GSH, PC2, and partially for PC3. Moreover, binuclear species CdxLy were identified for the series PC3-PC6 in an excess of Cd(II). Potentiometric and competition spectroscopic studies showed that the affinity of Cd(II) complexes increases from GSH to PC4 almost linearly from micromolar (log K7.4GSH = 5.93) to the femtomolar range (log K7.4PC4 = 13.39) and additional chain elongation does not increase the stability significantly. Data show that PCs form an efficient system which buffers free Cd(II) ions in the pico- to femtomolar range under cellular conditions, avoiding significant interference with Zn(II) complexes. Our study confirms that the favorable entropy change is the factor governing the elevation of phytochelatins' stability and illuminates the importance of the chelate effect in shifting the free Gibbs energy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Metallothioneins and related chelators. In Metal ions in life sciences; Sigel A., Sigel H., Sigel R., Eds.; Royal Society of Chemistry: Cambridge, U.K.; 2009.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

