Senescent accelerated prone 8 (SAMP8) mice as a model of age dependent neuroinflammation
- PMID: 33736657
- PMCID: PMC7977588
- DOI: 10.1186/s12974-021-02104-3
Senescent accelerated prone 8 (SAMP8) mice as a model of age dependent neuroinflammation
Abstract
Background: Aging and age-related diseases are strong risk factors for the development of neurodegenerative diseases. Neuroinflammation (NIF), as the brain's immune response, plays an important role in aged associated degeneration of central nervous system (CNS). There is a need for well characterized animal models that will allow the scientific community to understand and modulate this process.
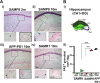
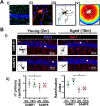
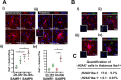
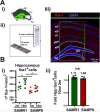
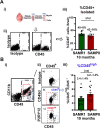
Methods: We have analyzed aging-phenotypical and inflammatory changes of brain myeloid cells (bMyC) in a senescent accelerated prone aged (SAMP8) mouse model, and compared with their senescence resistant control mice (SAMR1). We have performed morphometric methods to evaluate the architecture of cellular prolongations and determined the appearance of Iba1+ clustered cells with aging. To analyze specific constant brain areas, we have performed stereology measurements of Iba1+ cells in the hippocampal formation. We have isolated bMyC from brain parenchyma (BP) and choroid plexus plus meningeal membranes (m/Ch), and analyzed their response to systemic lipopolysaccharide (LPS)-driven inflammation.
Results: Aged 10 months old SAMP8 mice present many of the hallmarks of aging-dependent neuroinflammation when compared with their SAMR1 control, i.e., increase of protein aggregates, presence of Iba1+ clusters, but not an increase in the number of Iba1+ cells. We have further observed an increase of main inflammatory mediator IL-1β, and an augment of border MHCII+Iba1+ cells. Isolated CD45+ bMyC from brain parenchyma (BP) and choroid plexus plus meningeal membranes (m/Ch) have been analyzed, showing that there is not a significant increase of CD45+ cells from the periphery. Our data support that aged-driven pro-inflammatory cytokine interleukin 1 beta (IL-1β) transcription is enhanced in CD45+BP cells. Furthermore, LPS-driven systemic inflammation produces inflammatory cytokines mainly in border bMyC, sensed to a lesser extent by the BP bMyC, showing that IL-1β expression is further augmented in aged SAMP8 compared to control SAMR1.
Conclusion: Our data validate the SAMP8 model to study age-associated neuroinflammatory events, but careful controls for age and strain are required. These animals show morphological changes in their bMyC cell repertoires associated to age, corresponding to an increase in the production of pro-inflammatory cytokines such as IL-1β, which predispose the brain to an enhanced inflammatory response after LPS-systemic challenge.
Keywords: Aging; Brain myeloid cells; IL-1β; Inflammation; Microglia; SAMP8.
Conflict of interest statement
Authors declare that they have no competing interests.
Figures


References
-
- Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65(3):199–203. - PubMed
-
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45(2):208–212. - PubMed
-
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25(3):294–297. - PubMed
-
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429(6994):883–891. - PubMed
-
- Ximerakis M, Lipnick SL, Innes BT, Simmons SK, Adiconis X, Dionne D, et al. Single-cell transcriptomic profiling of the aging mouse brain. Nat Neurosci. 2019;22(10):1696–1708. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

