Astrocyte-specific hypoxia-inducible factor 1 (HIF-1) does not disrupt the endothelial barrier during hypoxia in vitro
- PMID: 33736658
- PMCID: PMC7977259
- DOI: 10.1186/s12987-021-00247-2
Astrocyte-specific hypoxia-inducible factor 1 (HIF-1) does not disrupt the endothelial barrier during hypoxia in vitro
Abstract
Background: Astrocytes (AC) are essential for brain homeostasis. Much data suggests that AC support and protect the vascular endothelium, but increasing evidence indicates that during injury conditions they may lose their supportive role resulting in endothelial cell activation and BBB disturbance. Understanding the triggers that flip this switch would provide invaluable information for designing new targets to modulate the brain vascular compartment. Hypoxia-inducible factor-1 (HIF-1) has long been assumed to be a culprit for barrier dysfunction as a number of its target genes are potent angiogenic factors. Indeed AC themselves, reservoirs of an array of different growth factors and molecules, are frequently assumed to be the source of such molecules although direct supporting evidence is yet to be published. Being well known reservoirs of HIF-1 dependent angiogenic molecules, we asked if AC HIF-1 dependent paracrine signaling drives brain EC disturbance during hypoxia.
Methods: First we collected conditioned media from control and siRNA-mediated HIF-1 knockdown primary rat AC that had been exposed to normoxic or hypoxic conditions. The conditioned media was then used to culture normoxic and hypoxic (1% O2) rat brain microvascular EC (RBE4) for 6 and 24 h. Various activation parameters including migration, proliferation and cell cycling were assessed and compared to untreated controls. In addition, tight junction localization and barrier stability per se (via permeability assay) was evaluated.
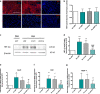
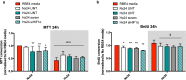
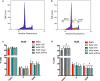
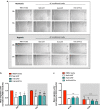
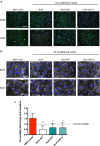
Results: AC conditioned media maintained both normoxic and hypoxic EC in a quiescent state by suppressing EC metabolic activity and proliferation. By FACs we observed reduced cell cycling with an increased number of cells in G0 phase and reduced cell numbers in M phase compared to controls. EC migration was also blocked by AC conditioned media and in correlation hypoxic tight junction organization and barrier functionality was improved. Surprisingly however, AC HIF-1 deletion did not impact EC responses or barrier stability during hypoxia.
Conclusions: This study demonstrates that AC HIF-1 dependent paracrine signaling does not contribute to AC modulation of EC barrier function under normoxic or hypoxic conditions. Thus other cell types likely mediate EC permeability in stress scenarios. Our data does however highlight the continuous protective effect of AC on the barrier endothelium. Exploring these protective mechanisms in more detail will provide essential insight into ways to prevent barrier disturbance during injury and disease.
Keywords: Astrocytes; Barrier stability; Endothelial activation; HIF-1; In vitro BBB; Primary culture.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Hertz L, Chen Y. Importance of astrocytes for potassium ion (K(+)) homeostasis in brain and glial effects of K(+) and its transporters on learning. Neurosci Biobehav Rev. 2016;71:484–505. - PubMed
-
- Ioannou MS, Jackson J, Sheu SH, Chang CL, Weigel AV, Liu H, et al. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell. 2019;177(6):1522.e14–1535.e14. - PubMed
-
- McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, et al. Tight junctions contain oligomeric protein assembly critical for maintaining blood–brain barrier integrity in vivo. J Neurochem. 2007;103(6):2540–2555. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

