Evaluation on immediate analgesic efficacy and safety of Kai-Hou-Jian spray (children's type) in treating sore throat caused by acute pharyngitis and tonsillitis in children: study protocol for a randomized controlled trial
- PMID: 33736674
- PMCID: PMC7977177
- DOI: 10.1186/s13063-021-05148-1
Evaluation on immediate analgesic efficacy and safety of Kai-Hou-Jian spray (children's type) in treating sore throat caused by acute pharyngitis and tonsillitis in children: study protocol for a randomized controlled trial
Abstract
Background: Acute pharyngitis and tonsillitis are common respiratory diseases for which children seek medical care. Their main clinical manifestation is sore throat which interferes with patients' quality of life. However, there is no proven effective or safe method to treat it. It is necessary to find an excellent strategy to reduce sore throat and reduce the burden of acute illness. We designed the randomized controlled trial with the characteristics of traditional Chinese medicine (TCM) to determine the clinical positioning of Kai-Hou-Jian spray (children's type) (KHJS) through evidence-based research. This trial aims to evaluate the immediate analgesic efficacy of KHJS on sore throat caused by acute pharyngitis and tonsillitis (wind-heat syndrome/heat exuberance in lung and stomach syndrome) in children and to observe its safety.
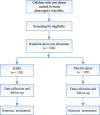
Methods/design: This is a prospective, multicenter, randomized, double-blind, parallel-group, placebo-controlled trial. It will include 240 children with acute pharyngitis/tonsillitis from 7 study sites across China. All participants are randomly assigned to two parallel treatment groups, one with KHJS and the other with placebo sprays, for 5 consecutive days. The primary outcome is the time of analgesic onset. Secondary outcomes include duration of analgesic effect, area under time curve of 0-3 h Wong-Baker FACES Pain Rating Scale (WBS) score (AUC0-3 h), rate of analgesic onset, rate of disappearance of sore throat, changes of WBS score (in days), effective rate of pharyngeal signs, and effective rate of TCM syndrome. The incidence of adverse events during the trial is the primary safety outcome. In addition, vital signs and laboratory tests before and after medication are monitored.
Discussion: To our knowledge, this will be the first clinical trial to explore the immediate analgesic efficacy of a Chinese patent medicine spray for acute pharyngitis/tonsillitis induced sore throat in children in a multicenter, randomized, double-blinded, parallel-group, placebo-controlled manner. Not only might it prove the efficacy and safety of KHJS in the treatment of sore throat caused by acute pharyngitis/tonsillitis in children, but it might also provide evidence for the treatment of acute sore throat with Chinese herbal medicine.
Trial registration: A multicenter, randomized, double-blind, very low-dose, parallel controlled trial for the immediate analgesic effect and safety of Kai-Hou- Jian spray (children's type) in the treatment of sore throat caused by acute pharyngitis and tonsillitis in children. Chinese Clinical Trial Registry ChiCTR2000031599 . Registered on 5 April 2020.
Keywords: Acute pharyngitis/tonsillitis; Kai-Hou-Jian spray; Randomized controlled trial; Sore throat; Traditional Chinese medicine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Efficacy and safety of Qi-Wei-Qing-Yan aerosol in treatment of acute pharyngitis (lung-stomach excess-heat syndrome): study protocol for a randomized controlled trial.Trials. 2016 Feb 19;17:99. doi: 10.1186/s13063-016-1217-4. Trials. 2016. PMID: 26896352 Free PMC article. Clinical Trial.
-
Efficacy and safety of Kegan Liyan oral liquid for patients with acute pharyngitis: A randomized, double-blinded, placebo-controlled, multi-center trial.Phytomedicine. 2024 Nov;134:155960. doi: 10.1016/j.phymed.2024.155960. Epub 2024 Aug 23. Phytomedicine. 2024. PMID: 39217655 Clinical Trial.
-
Safety and efficacy of a traditional herbal medicine (Throat Coat) in symptomatic temporary relief of pain in patients with acute pharyngitis: a multicenter, prospective, randomized, double-blinded, placebo-controlled study.J Altern Complement Med. 2003 Apr;9(2):285-98. doi: 10.1089/10755530360623400. J Altern Complement Med. 2003. PMID: 12804082 Clinical Trial.
-
[How to reduce the antibacterial load in the treatment of acute tonsillitis and pharyngitis? Possible tactics and practical approaches].Vestn Otorinolaringol. 2020;85(6):90-99. doi: 10.17116/otorino20208506190. Vestn Otorinolaringol. 2020. PMID: 33474925 Review. Russian.
-
[Tonsillitis and sore throat in childhood].Laryngorhinootologie. 2014 Mar;93 Suppl 1:S84-102. doi: 10.1055/s-0033-1363210. Epub 2014 Apr 7. Laryngorhinootologie. 2014. PMID: 24710788 Review. German.
Cited by
-
Pharmacological effects and mechanism of Kaihoujian Throat Spray (children's type) in the treatment of pediatric acute pharyngitis and tonsillitis.Heliyon. 2023 Jun 30;9(7):e17802. doi: 10.1016/j.heliyon.2023.e17802. eCollection 2023 Jul. Heliyon. 2023. PMID: 37539230 Free PMC article.
References
-
- Bridges-Webb C. Acute pharyngitis, tonsillitis and tonsillectomy. Aust Fam Physician. 1977;6(5):498–509. - PubMed
-
- GR Fleisher. Evaluation of sore throat in children. UpToDate. https://www.uptodate.com/contents/zh-Hans/evaluation-of-sore-throat-in-c.... Accessed 28 Apr 2020.
-
- Jan E Drutz. Acute pharyngitis in children and adolescents: symptomatic treatment. UpToDate. https://www.uptodate.com/contents/acute-pharyngitis-in-children-and-adol.... Accessed 28 Apr 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

