Magnetic resonance imaging and ultrasound for prediction of residual tumor size in early breast cancer within the ADAPT subtrials
- PMID: 33736679
- PMCID: PMC7977310
- DOI: 10.1186/s13058-021-01413-y
Magnetic resonance imaging and ultrasound for prediction of residual tumor size in early breast cancer within the ADAPT subtrials
Abstract
Background: Prediction of histological tumor size by post-neoadjuvant therapy (NAT) ultrasound and magnetic resonance imaging (MRI) was evaluated in different breast cancer subtypes.
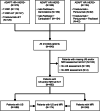
Methods: Imaging was performed after 12-week NAT in patients enrolled into three neoadjuvant WSG ADAPT subtrials. Imaging performance was analyzed for prediction of residual tumor measuring ≤10 mm and summarized using positive (PPV) and negative (NPV) predictive values.
Results: A total of 248 and 588 patients had MRI and ultrasound, respectively. Tumor size was over- or underestimated by < 10 mm in 4.4% and 21.8% of patients by MRI and in 10.2% and 15.8% by ultrasound. Overall, NPV (proportion of correctly predicted tumor size ≤10 mm) of MRI and ultrasound was 0.92 and 0.83; PPV (correctly predicted tumor size > 10 mm) was 0.52 and 0.61. MRI demonstrated a higher NPV and lower PPV than ultrasound in hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-positive and in HR-/HER2+ tumors. Both methods had a comparable NPV and PPV in HR-/HER2- tumors.
Conclusions: In HR+/HER2+ and HR-/HER2+ breast cancer, MRI is less likely than ultrasound to underestimate while ultrasound is associated with a lower risk to overestimate tumor size. These findings may help to select the most optimal imaging approach for planning surgery after NAT.
Trial registration: Clinicaltrials.gov , NCT01815242 (registered on March 21, 2013), NCT01817452 (registered on March 25, 2013), and NCT01779206 (registered on January 30, 2013).
Keywords: Breast cancer; Magnetic resonance imaging; Neoadjuvant therapy; Residual tumor size; Ultrasound.
Conflict of interest statement
MG received honoraria from AstraZeneca and travel support from Daiichi Sanyko.
OG has an ownership interest in WSG GmbH; received honoraria from Genomic Health, Roche, Celgene, Pfizer, Novartis, NanoString Technologies, and AstraZeneca; served in consulting/advisory role for Celgene, Exact Sciences, Lilly, MSD Brazil, Novartis pharma SAS, Pfizer Pharmaceuticals Israel, and Roche; and received travel support from Roche.
LU received honoraria, travel support and served in consulting/advisory role for Siemens Healthcare, Bayer Healthcare, and received research funding from Siemens Healthcare.
RW served in consulting/advisory role and received travel support from Agendia, Amgen, Aristo, AstraZeneca, Boeringer Ingelheim, Carl Zeiss, Celgene, Clinsol, Daiichi-Sankyo, Esai, Genomic Health, Glaxo Smith Kline, Hexal, Lilly, Medstrom Medical, MSD, Mundipharma, Nanostring, Novartis, Odonate, Paxman, Palleos, Pfizer, Pierre Fabre, PumaBiotechnolgogy, Riemser, Roche, Sandoz/Hexal, Seattle Genetics, Tesaro Bio, and Teva.
JA is an employee of Alcedis GmbH, Giessen, Germany.
SK has an ownership interest in WSG GmbH; served in consulting/advisory role for Roche, Genomic Health, Novartis, AstraZeneca, Amgen, Celgene, SOMATEX Medical Technologies, Daiichi Sankyo, pfm medical, Pfizer, MSD, Lilly, and Sonoscape; and received travel support from Roche, Daiichi Sankyo, and Sonoscope.
HF received honoraria and travel support and served in consulting/advisory role for Roche, Celgene.
MB received honoraria from AstraZeneca, Exact Sciences, Novartis, Pfizer, Roche, Teva, travel support from AstraZeneca, Celgene, Medac, Novartis, and Roche and served in consulting/advisory role for AstraZeneca, Exact Sciences, Novartis, Puma, and Roche.
BA received honoraria from Pfizer, Roche Pharma, Novartis Pharma, AstraZeneca, Amgen, Tesaro Bio Germany, PharmaMar, and Eisei; served in consulting/advisory role for Novartis Pharma, Roche Pharma, Pfizer, and Tesaro Bio; and received travel support from AstraZeneca, Amgen, Roche Pharma, Pfizer, Novartis Pharma, Tesaro Bio Germany, PharmaMar, and Eisei.
CKL has an ownership interest in Theraklion and Phaon Scientific; received honoraria from Roche, AstraZeneca, Celgene, Novartis, Pfizer, Lilly, Hexal, Amgen, SonoScape, Pfizer, Novartis, Roche, Genomic Health, Amgen, AstraZeneca, Riemser, Carl Zeiss MediTec, TEVA Pharmaceuticals Industries, Theraklion, Janssen-Cilag, GlaxoSmithKline, and LIV Pharma; served in consulting/advisory role for Roche, Novartis, Pfizer, Celgene, Phaon Scientific, Pfizer, Novartis, SurgVision, CarlZeissMeditec, Amgen, and Onkowissen; received research funding from Roche, Novartis, and Pfizer; and received travel support from Roche, Daiichi Sankyo, Novartis, Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, and Daiichi Sankyo.
NH has an ownership interest in WSG GmbH; received honoraria from Amgen, AstraZeneca, Genomic Health, Novartis, Pfizer, Pierre Fabre, Roche, and Zodiac Pharma; served in consulting/advisory role for Agendia, AstraZeneca, Celgene, Daiichi Sankyo, Lilly, Merck Sharp & Dohme, Novartis, Odonate Therapeutics, Pfizer, Pierre Fabre, Roche/Genentech, Sandoz, Seattle Genetics, and West German Study Group; and received research funding from Lilly, Merck Sharp & Dohme, Novartis, Pfizer, and Roche/Genentech.
CKK received honoraria from Bayer Healthcare.
UN has an ownership interest in WSG GmbH; received honoraria from Agendia, Amgen, Celgene, Genomic Health, NanoString Technologies, Novartis pharma SAS, Pfizer Pharmaceuticals Israel, Roche/Genentech, and Teva; served in consulting/advisory role for Genomic Health and Roche; received research funding from Agendia, Amgen, Celgene, Genomic Health, NanoString Technologies, Roche, and Sanofi; provided expert testimony for Genomic Health; and received travel support from Genomic Health, Pfizer Pharmaceuticals Israel, and Roche.
Figures


References
-
- Arnaout A, Lee J, Gelmon K, Poirier B, Lu FI, Akra M, et al. Neoadjuvant therapy for breast cancer: updates and proceedings from the seventh annual meeting of the Canadian Consortium for Locally Advanced Breast Cancer. Curr Oncol. 2018;25(5):e490. doi: 10.3747/co.25.4153. - DOI
-
- Croshaw R, Shapiro-Wright H, Svensson E, Erb K, Julian T. Accuracy of clinical examination, digital mammogram, ultrasound, and MRI in determining postneoadjuvant pathologic tumor response in operable breast cancer patients. Ann Surg Oncol. 2011;18(11):3160–3163. doi: 10.1245/s10434-011-1919-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

